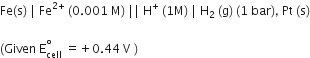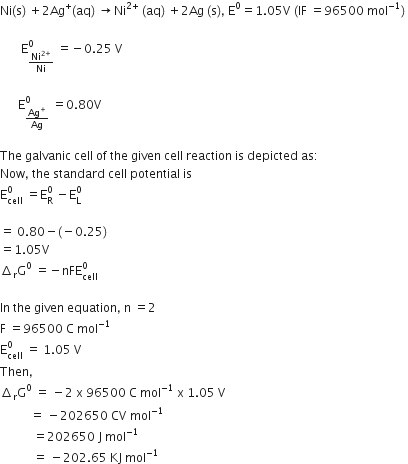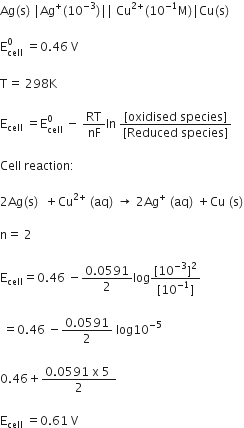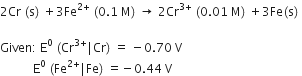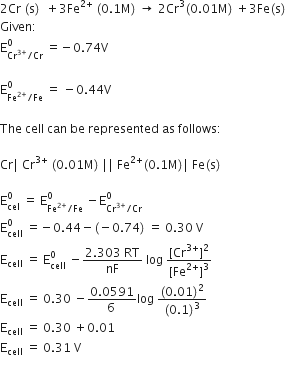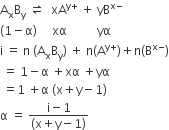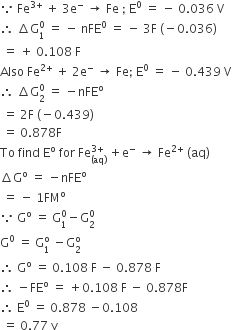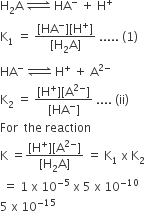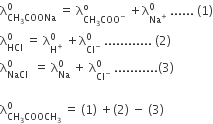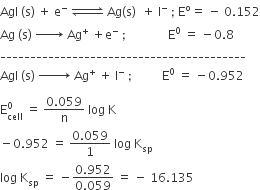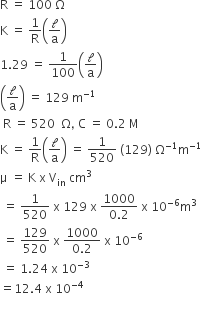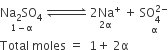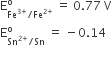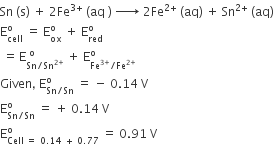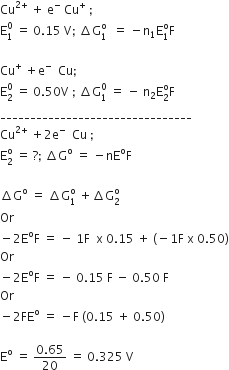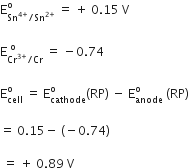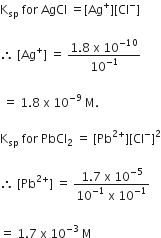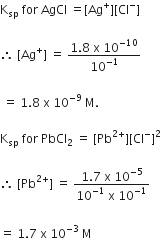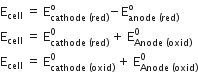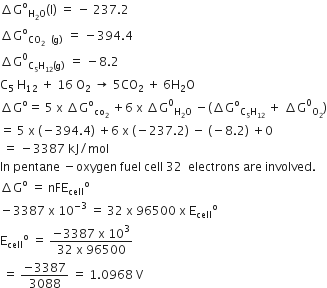The conductivity of a solution is directly proportional to number of ions present in a unit volume of the solution because current is carried forward by the ions. With dilution number of ions in unit volume decreases so that conductivity also decreases. Hence with dilution conductivity decreases.
Chemistry I Chapter 3 Electrochemistry
Sponsor Area
NCERT Solution For Class 12 Business+studies Chemistry I
How would you determine the standard electrode potential of the system Mg2+/Mg?
Use standard hydrogen electrode as anode and Mg2+ | Mg as a cathode we can measure the standard electrodepotential of systemMg2+ | Mg. Standard hydrogen electrode, represented by Pt(s), H2(g) (1 atm) | H+ (aq) and dip the electrode of Magnesium wire in a 1M MgSO4 solution .The standard hydrogen electrode is always zero.
Can you store copper sulphate solutions in a Zinc pot?
No. Because zinc is more reactive than copper and thus holes will be developed in zinc pot.
Cu2+(aq) + Zn(s) → Zn2+ (aq) + Cu(s)
Consult the table of standard electrode potentials and suggest three substance that can oxidize ferrous ions under suitable conditions.
oxidation of ferrous ion means :
Fe2+--> Fe3+ +e- ;
Any substance which standard electrode potential is more than that of Fe+3 /F+2 can oxidise ferrous ions.
(refer to the table given in book)
The EMF of the substance whose reduction potentials greater than 0.77V will oxidised ferrous ion.
for example Br2, Cl2,and F2 .
Calculate the potential of hydrogen electrode in contact with a solution whose pH is 10.
Calculate the emf of the cell in which the following reaction takes place:
Ni(s) + 2Ag+ (0.002 M) Ni2+ (0.160 M) + 2Ag(s)
Given that
or
The equation is also written as
or
= 1.05 V – 0.0295 x log 80
= 1.05 V – 0.0295 x 1.9031
= 1.05 V – 0.056 = 0.99 V.
Why does the conductivity of a solution decrease with dilution?
The conductivity of a solution is directly proportional to number of ions present in a unit volume of the solution because current is carried forward by the ions. With dilution number of ions in unit volume decreases so that conductivity also decreases. Hence with dilution conductivity decreases.
Suggest a way to determine the Λ°m value of water.
The molar conductivity of 0.025 mol L–1 methanoic acid is 46.1 S cm2 mol–1. Calculate its degree of dissociation and dissociation constant. Give λ°(H+) = 349.6 S cm2 mol–1 and λ° (HCOO– ) = 54.6 s cm2 mol–1.
Answer:

If the current of 0.5 ampere flows through a metallic wire for 2 hours, then how many electrons flow through the wire?
Current, I = 0.5 A
Suggest a list of metals that are extracted electrolytically.
Consider the reaction: Cr2O72– + 14H+ + 6e- → 2Cr3+ + 8H2O
What is the quantity of electricity in coulombs needed to reduce 1 mol of Cr2O72-?
Cr2O72– → 2Cr3+
2Cr6+ + 6e– → 2Cr3+
Write the chemistry of recharging the lead storage battery, highlighting all the materials that are involved during recharging.
Answer:
A secondary cell after use can be recharged by passing current through it in the opposite direction so that it can be used again.
Recharging: During charging process, hydrogen ions moves to cathode and sulphate ions to anode and the following reactions take place
At cathode:
PbSO4 + H2 → Pb + H2SO4
At anode:
PbSO4 + SO4 + 2H2O → PbO2 + 2H2SO4
Thus, during charging active materials namely Pb cut the cathode and PbO2 at the anode are formed. Sulphuric acid is formed and water is iconsumed. Due to this, the specific gravity of sulphuric acid increases and emf of the cell goes up to 2.2 volt.
Suggest two materials other than hydrogen that can be used as fuels in fuel cells.
Explain how rusting of iron is envisaged as setting up of an electrochemical cell.
At a particular spot of an object made of iron oxidation takes place and that spot behaves as an anode.
At anode:
Electrons released at anode spot moves through the metal and go to another spot on the metal and reduce oxygen in presence of H+ (which is believed to be available from H2CO3formed due to dissolution of carbondioxide from air into water. Hydrogen ion in water may also be available due to dissolution of other acidic oxides from the atmosphere). This spot behaves as a cathode with the reaction.
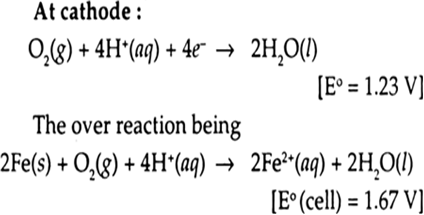
The ferrous ions are further oxidized by atmospheric oxygen to ferric ions which come out as rust in the form of hydrated ferric oxide (Fe2O3 x H2O).
What is thermodynamic efficiency of a cell?
The fuel cell thermodynamic efficiency is given by the ratio of the Gibbs function change to the Enthalpy change in the overall cell reaction. The Gibbs function change measures the electrical work and the enthalpy change is a measure of the heating value of the fuel.
Efficiency = (dG/dH)
Sponsor Area
Can we use copper vessel to store AgNO3 solution ?
No because copper is more reactive than silver thus it replace silver and formed product.
The reaction
Cu + 2AgNO3 → Cu(NO3)2 + 2Ag will occur.
Suggest a metal that can be used for cathodic protection of iron against rusting.
Aluminium or zinc or magnesium which are more electro + ve than iron can be used for cathodic protection of iron against rusting.
How does a fuel cell operate?
Galvanic cells that are designed to convert
the energy of combustion of fuels like hydrogen, methane, methanol, etc. directly into electrical energy are called fuel cells
Oxidation/combustion takes place at anode with the liberation of energy.
The electrode
reactions are given below:
Cathode: O2(g) + 2H2O(l) + 4e- -->4OH-(aq)
Anode: 2H2 (g) + 4OH-(aq) ---> 4H2O(l) + 4e-
Overall reaction being:
2H2(g) + O2(g) ---> 2 H2O(l )
State Kohlrausch’s law of independent migration of ions.
Kohlrausch examined Ëm° values for a number of strong electrolytes and observed certain regularities. He noted that the difference in Ëm° of the electrolytes NaX and KX for any X is nearly constant. For example
at 298 K:
Ëm°(KCl) – Ëm° (NaCl)= Ëm°(KBr) – Ëm°(NaBr)
= Ëm°(KI) – Ëm° (NaI) ≃ 23.4 S cm2 mol–1
and similarly it was found that
Ëm°(NaBr)– Ëm°(NaCl)= Ëm°(KBr) – Ëm°(KCl) ≃ 1.8 S cm2 mol–1
On the basis of the above observations he enunciated Kohlrausch
law of independent migration of ions
How is molar conductivity related to the degree of dissociation?
At any concentration c, if a is the degree of dissociation then it can be approximated to the ratio of molar conductivity Ëm at the concentration c to limiting molar conductivity, Ëm°. Thus we have
Λm and degree of dissociation (a) are related by the equation
How is molar conductivity related to the degree of dissociation?
Λm and degree of dissociation (a) are related by the equation
Given the two half cell reaction:
Anode: Cu(s) → Cu2+ (aq) + 2e–
Cathode: Ag+ (aq) + e– → Ag(s)
Write the galvanic cell for the net reaction.
The net cell reaction is
Cu(s) + 2Ag+ (aq) → Cu2+ (aq) + 2Ag(s)
and the galvanic cell is
Cu(s) |Cu2+ (aq) || Ag+ (aq) | Ag(s)
The electrode potentials of four elements A, B, C and D are 1.36 V - 0.32 V, 0.0 V and – 1.26 V respectively. Arrange these elements in the decreasing order of reactivity.
The reactivity decreases as the electrode potential increases, the correct order of decreasing order of reactivity is D > B > C > A .
What is it that ‘Al’ metal cannot be obtained by electrolysis of an aqueous solution of a salt of aluminium?
The reduction potential of aluminium is lower than that of water this means that it has lesser tendency to get reduced than water .
since water has higher reduction potential thus aluminium on electrolysis of an aqueous solution of a salt of aluminium.
How many Faradays of electricity are required to liberate 2 moles of hydrogen gas in electrolysis of aq. solution?
4H+ + 4e– → 2H2(g).
4 Faraday of electricity is required to liberate 2 moles of hydrogen gas.
In operation of a galvanic cell, at one of its electrodes oxidation takes place. What is the name of electrode and what is its polarity?
The name of the electrode is anode and it has -ve polarity.
What is cathodic protection?
It is a method of protecting a metal from corrosion by connecting it with another metal that is more easily oxidised.
for example zinc and aluminium used to protect iron.
What are the products obtained during electrolysis of CuSO4 using Cu electrodes?
The electrolysis of an aqueous solution of copper sulphate using copper electrodes (i.e. using active electrodes) results in transfer of copper metal from the anode to the cathode during electrolysis. The copper sulphate is ionised in aqueous solution.
CuSO4 - -> Cu2+ + S
The positively charged copper ions migrate to the cathode, where each gains two electrons to become copper atoms that are deposited on the cathode.
Cathode: Cu2+ + 2e → Cu
At the anode, each copper atom loses two electrons to become copper ions, which go into solution.
Anode: Cu → Cu2+ + 2e
The sulphate ion does not take part in the reaction and the concentration of the copper sulphate in solution does not change. The reaction is completed when the anode is completely eaten away. This process is used in electroplating
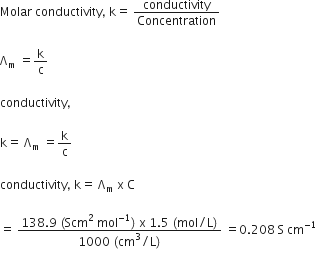
Give the unit of molar conductivity.
unit of conductivity can be given as :
Ohm–1 Cm–2 mol–1 or Scm2 mol–1.
What is the representation of the Daniell cell?
Daniell cell has Zn and Cu electrodes. Therefore, its representation is
Zn(s) | Zn2+ (aq) || Cu2+ (aq) + Cu(s)
Which solution will allow greater conductance of electricity, 1 M NaCl at 293 K or 1 M NaCl at 323 K?
1 M NaCl at 323 K will allow greater conductance of electricity as the ionic mobilities increase with increase in temperature.
Give an example of “fuel celIs”.
Galvanic cells that are designed to convert
the energy of combustion of fuels like hydrogen, methane, methanol,
etc. directly into electrical energy are called fuel cells.
H2—O2 fuel cell.
Why is it impossible to obtain the electrode potential for a single half cell.
This is because emf’s can only be measured for a completed circuit with two electrodes.
Write the equation showing the relation between standard free energy and standard cell potential?
The relation between satandard free energy and standard cell potential is given by
ΔG° = – nFE°cell.
How does fuel cell operate?
In fuel cell, chemical energy of fuel is converted into electrical energy.
Why is the equilibrium constant K related to only
E°cell and not Ecell?
This is because at equilibrium, Ecell becomes zero.
Sponsor Area
Explore the possibility of a substance that can be replace MnO2 is designing a dry cell.
MnO2 oxidises hydrogen into water.
Hence some other oxidizing agent can replace MnO2 in designing dry cell.
Predict the products of electrolysis of a dilute aqueous solution of sulphuric acid with inert electrodes.
Answer:
The electrolysis of an aqueous solution of sodium sulphate using inert electrodes produces hydrogen at the cathode and oxygen at the anode and a neutral solution of sodium sulphate remains unaltered by the electrolysis.
At the cathode:
2H+ + 2e– → H2
At the anode:
2H2O → O2 + 4H+ + 4e–
Write the electrode reaction taking place during the use of a dry cell.
Answer:
Anode: Zn + Zn2+ → 2e
Cathode: MnO2(s) + NH4+ + e– →MnO(OH) (s) + NH3 (aq)
Write an expression to relate molar conductivity of an electrolyte to its degree of dissociation?
Answer:
Degree of dissociation:
Why is it not possible to measure the voltage of an isolated reduction half reaction?
The reduction half reaction cannot take place alone.
Arrange the following metals in the order in which they displace each other?
Al, Cu, Fe, Mg, Zn
reactivity order of these metal are :
Mg > Al > Zn > Fe > Cu.
What is the basis of obtaining electrical energy in fuel cell?
In fuel cell, the chemical energy of fuel is converted into electrical energy.
Name a metal that can be used in the cathode protection of iron. Is it more active or less active than iron?
Since Zinc. It is more reactive than iron. thus it can be used in the cathode protection.
What is the standard electrode potential of NHE?
The standard electrode potential of normal electrode potential is Zero.
What is the EMF of the cell when the cell reaction attains equilibrium?
When the cell reaction attains equilibrium the EMF of the cell Zero.
What is the effect of temperature on the metallic conductance?
The metallic conductance decreases with increase of temperature and vice-versa.
For the cell:
Sn(s) + Sn2+ (aq) || H+ (aq) | H2(g) || Pt.
Write the electrode reactions.
Answer:
At anode: Sn(s) →Sn2+ (aq) + 2e–
At cathode: 2H+ (aq) + 2e– → H2(g)
To deposit 1 mole of aluminium from an aqueous solution of A12(SO4)3. What is the amount of electricity (in coulombs) required?
Q = n(e-) x F
For deposition of one mole of aluminium (96500 x 3) coulombs are required.
Express mathematically the relationship among the resistance (R), specific conductivity and cell constant.
Answer:
Specific conductivity =
What is cell constant? What are its units?
The quantity l/A is called cell constant denoted by the symbol, G*.
It depends on the distance between the electrodes and their area of cross-section and has the dimension of length–1 and can be calculated
if we know l and A.
What is the effect of dilution on equivalent conductance of solution?
Equivalent conductance increase with dilution. Increases with dilution and finally attains a limiting value of infinite dilution.
Name any two metals. Which can be used for cathodic protection of iron?
Zinc and aluminium are used for as cathodic protection of iron.
Which equation give the relationship between equivalent or molar conductance and concentration of a strong electrolytes?
b = it is constant which depend upon nature of solvent and temperature .
c= concentration
What are the units of specific conductance?
the units of specific conductance is given by
Ohm–1 metre–1 = Ω–1 m–1 = Sm–1.
How does cell constant changes with the concentration of the solution in the conductivity cell.
Cell constant is independent of the concentration of the electrolytic solution in the conductivity cell.
What are conductors?
.
The substances which allow the passage of electricity through them are called conductors.
for example copper .
What are electrolytes?
Those compounds which dissociate in to ions in aqueous solution or in molten state are called electrolytes. example NaCl, KCl etc
How many Faradays of electricity are required to liberate 2 moles of hydrogen gas in electrolysis of solution?
H2(g) → 2 H+(aq) + 2 e-
thus
2 Faradays of electricity are required to liberate 2 moles of hydrogen gas.
What is meant by Faraday constant?
It is total charge on 1 mole of electrons. It is unit of charge.
What does the standard electrode potential of a metal being negative (EZn2+/Zn = -0.763 V) indicate?
The negative value of reduction electrode potential indicates that the oxidation will take place at zero electrode.
Arrange the following metals in the order in which they displace each other from the solution of their salts Al, Cu, Fe, Mg and Zn.
Mg> Al> Zn> Fe> Cu is decreasing order of their reactivity.
What is the efficiency of fuel cell? What is the use of fuel cell?
The efficiency of fuel cell is 83.3%. Fuel cell is used in space vehicles.
Write the correct representation of cell:
2Cr(s) + 3Cd2+ (aq) → 2Cr3+ (aq) + 3Cd(s)
The correct representation of cell:
Cr(s) | Cr3+ (aq) || Cd2+ (aq) | Cd(s)
What is the role of the salt bridge in a voltaic cell?
A salt bridge allows the flow of ions to maintain a balance in charge between the oxidation and reduction vessels while keeping the contents of each separate. With the charge difference balanced, electrons can flow once again, and the reduction and oxidation reactions can proceed.
What is the effect of dilution on equivalent conductance of solution?
Increases with dilution and finally attains a limiting value of infinite dilution.
How is molar conductivity is related to molarity of electrolyte solutions?
Molar conductivity =
For the electrochemical reaction:
Zn2+(aq) + Cu(s) → Zn(s) + Cu2+(aq)
[E°cell = – 1.10 V]
What does the negative value of E°cell indicate?
The reaction is non-spontaneous or cell is not working.
Why is not possible to determine for weak electrolytes by extrapolation?
For weak electrolytes do not increase linearly with dilution like strong electrolytes.
Sponsor Area
What is the use of platinum foil in the hydrogen electrode?
It is used for inflow and outflow of electrons.
Why does a mercury cell give a constant voltage throughout its life?
This is because the electrolyte KOH is not consumed in the reaction.
Rusting of iron is quicker in saline water than in ordinary water. Why?
Rusting of iron is an oxidation process i.e. removal of electron.The electrolytes present in saline water help in forming the cells.
When a solution containing zinc ions and silver ions is electrolysed, silver is deposited at cathode. Why?
The discharge (reduction) potential of silver is less than that of zinc.
This is because these metals are less reactive than hydrogen and are lower down in the reactivity series. Reactive ions stay in solution and do not form atoms because they are more stable than the atoms.
What value for standard emf of normal hydrogen electrode has been assigned?
Zero value has been assigned for standard emf of normal hydrogen.
Write Nernst equation for single electrode potential.
for a single electrode potential
Why is the equilibrium constant K related to only E°cell and not Ecell?
It is because Ecell at equilibrium is 0 volt.
What is the basis of obtaining electrical energy in fuel cells?
Cells that are designed to convert the energy of combustion of fuels like hydrogen, methane, methanol, etc. directly into electrical energy are called fuel cells.
Combustion of fuels such as hydrogen, CO and CH4 is the basis of obtaining electrical energy in fuel cells.What will happen to the voltage if a salt bridge is removed between the half cells?
The purpose of a salt bridge is not to move electrons from the electrolyte, rather to maintain charge balance because the electrons are moving from one half cell to the other. The electrons flow from the anode to the cathode thus if a salt bridge is removed between the half cells, Voltage becomes zero.
The standard reduction potential values of three metallic cation X, Y, Z are 0.52, – 3.03 and – 1.18 V respectively. What will be the order of reducing power of the corresponding metals ?
The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The more positive the potential is the more likely it will be reduced.
Y > Z > X.
What is the effect of an increase in the concentration of zinc ions on the electrode potential of zinc electrode for which E° equals – 0.76 V?
According to Nernst equation, the electrode potential of zinc electrode will increase with the increase in the concentration of zinc ions.
What is a primary cell? Give an example.
Answer:
A primary cell is that electrochemical cell, which cannot be recharged and the chemicals are to be replaced in it regularly. A Leclanche cell is an example of a primary cell.
Express the relation between conductivity and molar conductivity of a solution.
where M is molarity of a solution.
Explain with examples the terms weak and strong electrolytes. How can these be distinguished?
(i) Weak electrolytes : An electrolyte that ionizes partially in solution is called a weak electrolyte. The solution formed contains ions which are in equilibrium with un-ionised molecules, e.g., acetic acid dissolves in water to form H3O+ and CH3COO+ ion. The solution contains H3O+ (hydronium ion), CH3COO– (acetate ion) and unionised CH3COOH molecules.
The degree of ionisation of a weak electrolyte is much less than 1. These have low values of molar conductivities at high concentration. Degree of ionisation and molar conductivity both increases with dilution.
(ii) Strong electrolyte : An electrolyte which is almost completely ionised in solution is called a strong electrolyte. The degree of ionisation of a strong electrolyte is 1 or 100% (or nearly so). The solution formed contains ions which are in equilibrium with solid form of strong electrolyte.
Strong electrolyte | Weak electrolyte |
1. These have higher molar conductivities at all concentrations. 2. λ°m values increase very slightly with dilution. 3. Degree of ionisation is very high at all concentration i.e., almost fully ionized. 4. Most of the salts like NaCl, KCl, NaNO3, BaCl2 and mineral acids like HCl, H2SO4, HNO3 and NaOH, KOH etc are common examples of strong electrolytes | 1. These have much lower conductivities at high concentration. 2. λ°m values increase sharply with dilution. 3. Degree of ionisation is very low at high concentration and increases with dilution. 4. Salts like ammonium acetate, acetic acid, aq NH4OH, aqueous CO2 and organic acids and bases are common examples of weak electrolytes. |
Predict the products of electrolysis in each of the following:
(i) An aqueous solution of AgNO3 with silver electrodes.
(ii) An aqueous solution of AgNO3 with platinum electrodes.
(iii) A dilute solution of H2SO4 with platinum electrodes.
(iv) An aqueous solution of CuCl2 with platinum electrodes.
At cathode: Ag+ are preferably discharged as compared to H+ as its reduction potential is higher
At anode: Silver electrode (being reactive) dissolves to produce Ag+
As the electrodes are inert, these do not take part in the reaction. At cathode, Ag+ ions are discharged in preference to H+ ions and OH– are discharged in preference to NO3– ions at anode
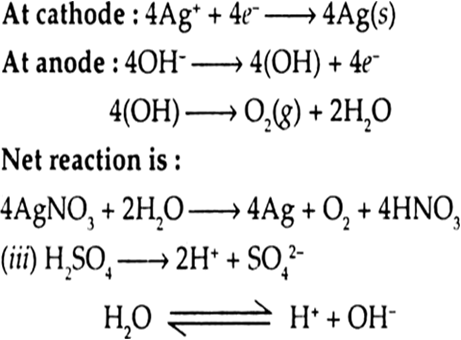
Here, only H+ are discharged at cathode as there is no other positive ion. Out of OH– and SO42– ions, OH– ions are discharged at anode preferably
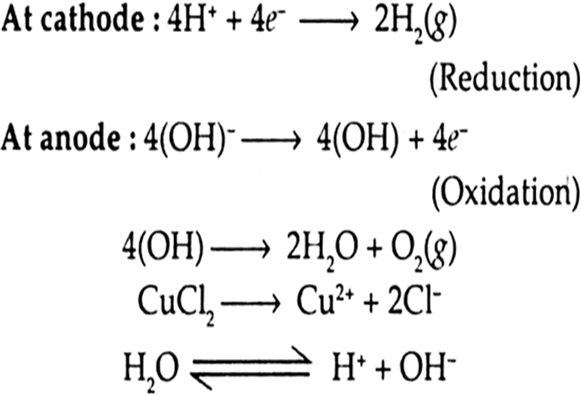
The platinum electrodes are inert and do not take part in the reaction. The following reactions occur at platinum (inert) electrodes.
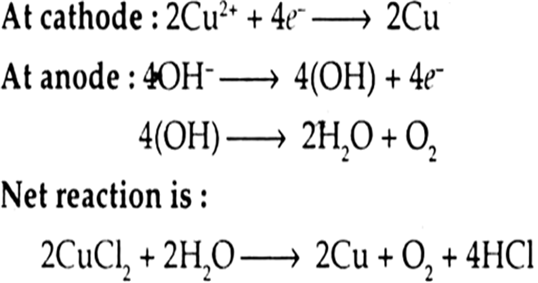
Depict the galvanic cell in which the reaction
Zn(s) + 2Ag+(aq) → Zn2+(aq) + 2Ag(s) takes place. Further show:
(i) Which of the electrode is negatively charged?
(ii) The carriers of the current in the cell.
(iii) Individual reaction at each electrode.
The electrochemical cell can be depicted as

(ii) Electrons move from anode (zinc electrode) to cathode (silver electrode) in the external circuit. Zinc ions go into solution at anode and Ag+ ions get deposited at cathode. Thus electrons in the external and metal ions in the internal circuit act as carrier of current in.
(iii) Overall reaction is obtained by anode and cathode reactions.
Zn(s) + 2Ag+(aq) → Zn2+(aq) + 2Ag(s)
Following two reactions can occur at cathode in the electrolysis of aqueous sodium chloride:
Na+ + e– → Na(s) 2H2O(l) + 2r → H2(g) + 2OH–(aq)
E°red = – 0.83 V
Which reaction takes place preferentially and why?
We know from electrochemical series and the standard reduction electrode potential that higher the reduction potential of a species, there is increasing tendency for its reduction to occur. Here, the standard reduction potential of water is greater than that of sodium ion, so reduction of water takes place in preference to that of sodium ion and H2 is liberated at cathode.
Iron does not rust even if zinc coating is broken in galvanized iron pipe. Explain.
Zinc is more reactive than iron, it loses electron more readily as compared to iron. In galvanized iron object, zinc acts as anode and does not allow the iron to lose electron, i.e. makes it a cathode. So long zinc is there on surface, it reduces to Fe2+ (if any formed) to iron back.
Explain why:
(i) E° for Mn3+ / Mn2+ couple is more positive than that Fe3+/Fe2+. (At. No. Mn = 25, Fe = 26).
(ii) Ce3+ can be easily oxidised to Ce4+ (At. No. Ce= 58).
Answer:
(i) Mn2+ is more stable than Mn3+ because Mn+2 has exactly half-filled orbitals, while Fe+2 after losing one e– half-filled orbitals.
(ii) Ce+4 achieve inert gas structure of Xenon, to require extra stability.
With the halp of ionic equations describe what happen: When
(i) pH of a solution of dichromate ions is raised.
(ii) Potassium manganate is electro-chemically oxidised.
when pH is increased, i.e., solution is more basic, orange coloured dichromate ion change to yellow coloured chromate ion.
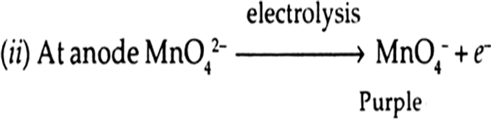
When potassium manganate is electro-chemically oxidised i.e., undergoes, electrolytic oxidation, it forms purple colour KMnO4.
The standard reduction potential values of three metal cations Xa+, Yb+ and Zc+are + 0.52, – 3.03, – 1.18 V respectively. Arrange the corresponding metals, in the order of their increasing reducing power.
The Standard reduction potential is the tendency for a chemical species to reduced and is measured in volts at standard condition the more is positive the potential is more likly it will reduced.
Yb+ > Zc+ > Xa+.
Explain with the help of a diagram the effect of change in concentration of solution on the molar conductance of (i) a weak electrolyte, (ii) a strong electrolyte.
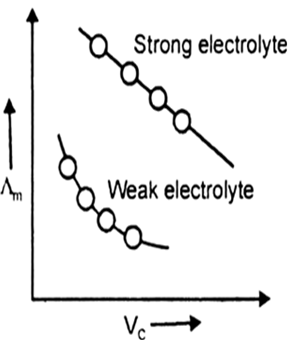
Λm increase a little in strong electrolyte because numbers of ions do not increase appreciably, only mobility of ions increases. Λm increases sharply in weak electrolyte because both number of ions and mobility of ions increases.
State two advantages of H2O fuel cell over ordinary cell.
Answer:
The advantage of water fuel cell over ordinary cell is,
(i) It has high-efficiency and eco-friendly.
(ii) The H2O produced can be used by astronauts for drinking purposes.
What happens during corrosion of a metal? State the electrochemical basis of corrosion of iron.
Answer:
Corrosion slowly coats the surfaces of metallic objects with oxides or other salts of the metal.
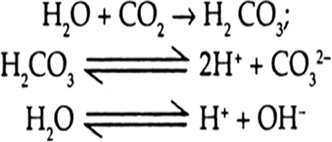
Iron in contact with the dissolved CO2 and O2 undergoes oxidation as follows:
Fe → Fe2+ + 2e– E°oxidation = + 0.44 V
The elctrons lost by iron are taken up by the H+ ions present on the surface of the metal which were produced by the dissociation of H2CO3 and H2O.
are -0.25 V and + 0.34 respectively at 298 K. Formulate the self operating galvanic cell for this electrode pair. What reaction takes place in its operation? How is the ΔG° for this reaction related to the cell e.m.f.?
we have given that
Relationship between and cell e.m.f.
here n=2
F=96500
E0cell= 0.59
plug in above equation we get
=-2 x 96500 x 0.59 =-113870 Kj/mol
How does molar conductance of a strong electrolyte vary with its concentration in solution?
At low concentrations, the molar conductance of strong electrolyte can be expressed by the relationship.
where
Λm = molar conductance at concentration C.
Λm = molar conductance at infinite dilution.
K = constant
C = concentration of solution on molar scale.
How does molar conductance of a weak electrolyte vary with its concentration in solution?

What do you understand by standard potential of a half-cell? How is the electrode potential of a half-cell determined?
Standard half-cell potential E° : If the ionic species have concentration of mol dm–3and pressure of the gaseous species is 1 atm (101.325 KPa), than half-cell potential is called as standard half-cell potential. The temperature being 298 K.
Absolute value of half-cell potential cannot be determined experimentally. However, its value relative to reference electrode can be determined.
Reference electrode 11 given half-cell. E°cell can be measured experimentally using a potentiometer.![]()
Knowing the standard reduction potential of the reference electrode E°half-cell can be found out.
Define molar conductivity of an electrolytic solution. Mention the effect of temperature on molar conductivity.
where Am = Molar conductivity.
k = Specific conductivity. Cm = Concentration in molar per litre.
Effect of temperature on molar conductivity. The speed of movement of ions increases with an increase in temperature. Therefore, molar conductivity increases with temperature.
Write anode and cathode reactions from electrolysis of water. How much charge will be transported for the decomposition of 3.6 g of H2O? [F = 96500 C mol–1]
At cathode:
At anode:
36 g of requires
3.6 of
What is the Nernst equation for the Potential of an electrode? Can Nernst equation be applied to the cell relation ? Apply this equation to a general reaction.
Nernst shows that for the electrode reaction:
the electrode potential at any concentration measured with respect to standard hydrogen electrode can be represented by:
but concentration of solid M is taken as unity as we have
R is gas constant (8.314 JK–1 mol–1),
F is Faraday constant (96487 C mol–1), T is temperature in kelvin and [Mn+] is the concentration of the species, Mn
The Nernst equation of this electrode
Instead of activity, we can take molar concentration.
For pure solid and liquid molar concentration is taken as unity.
Yes, Nernst equation can be applied to the cell reaction.
Explain Kohlrausch’s law of independent migration of ions. Mention one application of Kohlrausch’s law.
where v+ and v– are number of cations and anions per formula of electrolyte e.g.,
Λ∞ CaCl2 = λ∞ (Ca2+) + 2 λ∞ (CI–)
Λ = KCl = λ∞ (K+) + λ∞ (CI–)
Uses 1. It is used to find molar conductivity of weak electrolyte at infinite dilution which
cannot be obtained by extrapolation.
2. It is used to calculate degree of dissociation of weak electrolyte at a particular concentration.
Degree of dissociation
where Λem is molar conductivity of weak electrolyte at a particular concentration and Λemis molar conductivity of weak electrolyte at infinite dilution.
How does molar conductivity vary with concentration for (i) weak electrolyte and for (ii) strong electolyte? Give reasons for these variations.
(i) Weak electrolytes: When the concentration of weak electrolyte becomes very low, its degree of ionisation rises sharply. There is sharp increase in the number of ions in the solution. Hence, the molar conductivity of a weak electrolyte rises steeply at low concentration.
(ii) Strong electrolytes: The molar conductivity of a strong electrolyte decreases slightly with the increase in concentration. This decrease is due to the increase in interionic attractions as a result of greater numbr of ions per unit volume. With dilution, the ions are far apart, inter ionic attractions become weaker and conductance increases.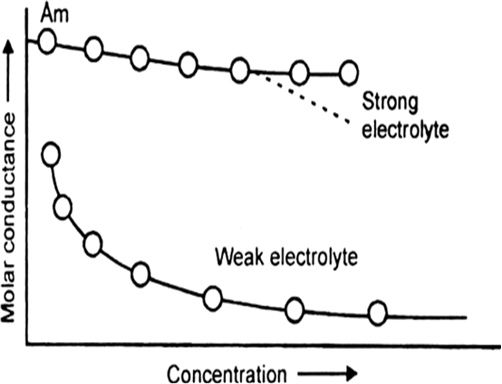
State and explain Faraday's law of electrolysis?
State and explain Faraday's law of electrolysis?
or It
or W = ZIt
where W is the mass of substance produced at an electrode.
I is current in amperes
t is time in seconds for which current is passed.
Z is electro-chemical equivalent of substance.
Faraday's second law: It states that the masses of different substances liberated or dissolved by the same amount of electricity passed is directly proportional to their chemical equivalents. Or in other words “the same quantity of electricity will produce or dissolve chemically equivalent quantities of all substances.”
Mathematically,
Thus, we can say that the same quantity of electricity is required to produce one equivalent of any substance. It is called Faraday, F. It is equal to 96500 coulombs, and is equal to the charge on one mole of electrons.
Show that the electrical work obtainable from a galvanic cell is given by the expression.
For reaction occurring in a electrochemical cell whose electrodes differ in potential by Ecell, the work done when amount of charge nF is transferred is given by
∴
Under standard state conditions
ΔG° = – n FE°cell.
What are fuel cells? Write the reaction of a oxygen hydrogen fuel cell.
Fuel cell: A galvanic cell in which the reactants are continuously fed into the cell and the products are continuously removed is called a fuel cell.
The most important fuel cell is hydrogen oxygen fuel cell.
The reactions taking place in this fuel cell are
Cathode: O2(g) + 2H2O(l) + 4e–--->4OH–(aq)
Anode: 2H2 (g) + 4OH–(aq)---> 4H2O(l) + 4e–
Overall reaction being:
2H2(g) + O2(g) ---> 2H2O(l )
What is a mercury cell? Give the electrode reactions?
Mercury cell is a new type of dry cell. It consists of
Anode: Zinc-mercury.
Cathode: Paste of HgO and carbon
Electrolyte: Paste of ZnO in KOH.
The reactions are:
Anode:
Zn (amalgam) +
At cathode:
Overall reaction:
What is the effect of change in (a) concentration, (b) temperature on the electrode potential of a given half-cell?
The Nernst equation is
From the equation, E ∝ T and E ∝ [Mn+]
(a) When the concentration of Mn+ increases, the half-cell potential increases and vice-versa.
(b) When temperature increases, the half-cell potential also increases.
Sponsor Area
Iron does not rust even if the zinc coating is broken in a galvanised iron pipe but, rusting occurs much faster if the tin coating over iron is broken. Explain?
Zinc is more electro-positive than iron. Therefore, as long as zinc is there on the iron pipe, zinc acts as anode and the iron as cathode. As a result, rusting of iron is prevented.
On the other hand, tin is less electro-positive than iron. Therefore, when tin coating over iron gets broken, iron acts as anode and gets oxidised. Thus even when tin is there, the exposed iron gets rusted.
Define the terms equivalent and molar conductivity. What are their physical signific-ance?
Equivalent conductivity : The conductivity of a volume (V) of a solution containing one equivalent of electrolyte placed between two electrodes separated by unit distance apart and of large enough area of cross-section to hold the entire volume (V) is called equivalent conductance. It is denoted by Λeq.
Λeq = k x V
where k = Specific conductance
V = Volume of solution containing one equivalent of electrolyte.
Molar conductivity : It is the conductivity of volume (V) of a solution containing 1 mole of a dissolved electrolyte place between two electrodes separated by unit distance apart and of enough area of cross-section to hold the entire volume V. It is denoted by Λm.
Λm= k x V = k / V where V = Volume of solution Containing 1 mole of electrolyte C = molarity of solution k = Specific conductivity.
How is molar conductivity related to concentration of an electrolyte? How will you explain a weak and a strong electrolyte based on their conductivity values?
conductivity and molar conductivity change with the concentration of the electrolyte. Conductivity always decreases with decrease in concentration both, for weak and strong electrolytes.
Molar conductivity varies with the concentration of electolytes.
Here b = Experimental constant
Λ∞m = Molar conductivity at infinite solution.
The plot of Λ∞m versus would give a straight line.
What is corrosion? What are the factors which effect corrosions? CO2 is always present in natural water. Explain its affect (increases, stops or no effect > on rusting of Fe.)
Corrosion slowly coats the surfaces of metallic objects with oxides or other salts of the metal. The rusting of iron, tarnishing of silver, development of green coating on copper and bronze are some of the
examples of corrosion.
Carbon dioxide reacts with water:
CO2 + H2O --> HCO3- + H+
As the concentration of CO2 increases, so does the concentration of the H+ ion. This ion then react with Fe in metals: Fe + 2H+ --> 2H (atom) + Fe2+ As corrosion proceeds, the ferrous ions produced react with the bicarbonate ions to form ferrous carbonate which precipitated as a scale
What is a salt bridge? What is its significance?
Salt bridge. A salt bridge consists of a saturated solution of NH4NO3 or KCl mixed with gelatin or agar jelly filled in a glass tube bent according to the requirement of the experiment.
Significance:
(i) Salt bridge prevents mixing of two electrolytes.
(ii) Prevents junction potential.
(iii) Maintains electrical neutraility.
What is corrosion? Describe the electrochemical phenomenon of rusting of iron.
Corrosion is the process of slowly eating away of the metal due to attack of the atmospheric gases on the surface of the metal resulting into the formation of compounds such as oxides, sulphides, carbonates, etc.
The corrosion of iron is called rusting.
According to theory of rusting, impure iron surface behaves as a small electrochemical cell in the presence of water containing dissolved oxygen or CO2.
The pure iron acts as anode and impure surface as cathode.
At Anode : Iron atom undergo oxidation spontaneously forming Fe2+ ion.
Fe → Fe2+ (aq) + 2e- E°cell = – 0.44 V
Fe2+ ions move into solution and electrons into cathodic area where they are picked up by H+ ions of the solution.
At cathode:
H+ ions are produced by secondary reaction either from H2O or from H2CO3 (CO2 + H2O)
H2O ---->H+ + OH–
H2CO3------> H+ + HCO3–
The overall reaction of the corrosion cell may be represented as:
The Fe2+ ions move through water and come at the surface where these are further oxidized into Fe3+ ions by atmospheric oxygen to form hydrate ferric oxide known as rust, Fe2O3.xH2O.
Explain why electrolysis of aqueous solution of NaCl gives H2 at cathode and Cl2 at anode. Write overall reaction.
At cathode: Both Na+ and H+ ions are present near the cathode. But the discharge potential of H+ is lower than that of Na+ ion. So H+ ions are discharged in preference to Na+ ions.
Thus H2 gas is liberated at the cathode and Na+ ions remain in the solution.
At the anode: Both Cl– and OH– ions are present near the anode. As the discharge potential of Cl– ions is lower than that of OH- ions, so Cl– ions are discharged in presence to OH– ions.
Thus Cl2 is liberated at anode and OH– ions remain in the solution.
The overall reaction is:
NaCl(aq) + H2 O(l) → Na+ (aq) + OH– (aq)
The following curve is obtained when molar conductivity λm (y-axis) is plotted against the square root of concentration C1/2 (x-axis) for two electrolytes A and B.
(a) What can you about the nature of the two electrolytes A and B.
(b) How do you account for the increase in molar conductivity λm for the electrolytes A and B on dilution.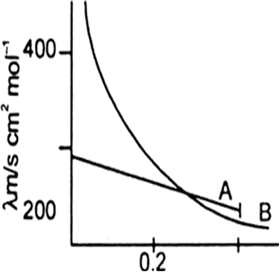
(a) A is strong electrolyte and B is weak electrolyte.
(b) The molar conductivity of a strong electrolyte:
(A) increase slightly as the concentration increased. This is because greater inter ionic attractions retrad the motion of the ions therefore molar conductivity decreases.The molar conductivity of a weak electrolyte.
(B) increase with decrease in the concentration of the solute in solution. The increase is very rapid at lower concentrations. The increase in the molar conductance with dilution is due to an increase in the effective degree of ionisation (α) at lower concentration and release of more full ions in the solution.
How is the standard free energy change related to
(i) emf of a galvanic cell related to the reaction.
(ii) equilibrium constant of the reaction in equilibrium state?
(i) The relation between free energy change and emf of a galvanic cell of the reaction is given as
ΔG = – nFEcell
(ii) The relation between free energy change and equilibrium constant of the reaction is given by
ΔG = – 2.303 RT log Kc.
Prove that the free energy (ΔG) and the emf of an electro-chemical cell are related by
ΔG = – nFE0.
The change in free energy is equal to the useful work done in a reversible process at constant temperature and pressure
ΔG = Wnet ...(i)
In an electrochemical cell and work obtained is equal to the charge transferred multiplied by the potential difference:
Welectrical = – nFE ...(ii)
The negative sign appears because the work is done by the charge. In equation (ii) n is the moles of electrons gained or lost in redox reaction and F is the Faraday constant.
In a cell when only electric work is done, then
Wnet= WeIectrical = – nFE ...(iii)
From relation (i) and (iii), we have
ΔG = – nFE
or ΔG = – mFE°cell.
What are fuel cells? Write reaction of a oxygen-hydrogen fuel cell. Write two advantages of the use of a hydrogen-oxygen fuel cell.

Hydrogen-oxygen fuel cell: The cell consists of three compartments separated from one another by porous electrode. The hydrogen gas is fed into one compartment and the oxygen gas is fed into another compartment. These gases then diffuse slowly through the electrodes and react with an electrolyte that is in the central compartment. The electrodes are made of a conducting material, such as graphite, with a sprinkling of platinum to act as a catalyst, and the electrolyte is an aqueous solution of a base. The reactions are
Cathode: O2(g) + 2H2O(l) + 4e–-----> 4OH–(aq)
Anode: 2H2 (g) + 4OH–(aq)-----> 4H2O(l) + 4e–
Overall reaction being:
2H2(g) + O2(g) ----->2H2O(l )
Advantages: (i) Fuel cells are efficient and free from pollution.
(ii) The only product in the reaction of fuel cell is water which can be removed and the astronauts of a spacecraft can drink it.
Write the cell reactions which occur in lead storage battery (i) when the battery is in use and (ii) when the battery is on charging.
The cell reactions when the battery is in use are given below:
Anode:
Cathode:
i.e., overall cell reaction consisting of cathode and anode reactions is:
ii) Recharging a lead-acid cell:
- is a non-spontaneous redox reaction (E(redox) is negative), that is, an electrolytic process
- requires an input of slightly more than 2 volts per cell to drive the spontaneous reactions in the reverse direction
- converts electrical energy back into chemical energy which is stored in the lead, lead dioxide and sulfuric acid in the cell
At Anode:
The conductivity of 0.20 M solution of KCl at 298 K is 0.0248 s cm–1. Calculate its molar conductivity.
Molarity of solution, M = 0.20
conductivity, i.e., specific conducitivity = k = 0.248 s cm–1 = 2.48 x 10–2 ohm–1cm–1
Thus, molar conductivity,λm = 124.0 s cm2 mol–1
How much charge is required for the following reductions:
1 mol of Al3+ to Al?
How much charge is required for the following reductions:
1 mol of Cu2+ to Cu?
How much charge is required for the following reductions:
1 mol of MnO4– to Mn2+?
Here n= 5
How much electricity in terms of Faraday is required to produce
20.0 g of Ca from molten CaCl2?
How much electricity in terms of Faraday is required to produce
40.0 g of Al from molten Al2O3?
or
How much electricity is required in coulomb for the oxidation of
1 mol of H2O to O2 ?
How much electricity is required in coulomb for the oxidation of
1 mol of FeO to Fe2O3
A solution of Ni(NO3)2 is electrolysed between platinum electrodes using a current of 5 amperes for 20 minutes. What mass of Ni is deposited at the cathode?
Three electrolytic cells A, B and C containing solutions of ZnSO4(zinc sulphate), AgNO3 (silver nitrate) and CuSO4 (copper sulphate), respectively are connected in series. A steady current of 1.5 ampere was passed through them until 1.45 g of silver is deposited at the cathode of cell B. How long did the current flow? What mass of copper and zinc were deposited? (Atomic masses: Ag = 108, Zn = 65.4, Cu = 63.5, all in amu).
108 g of silver is deposited at cathode when 1 mol of electrons are passed or 108 g of silver deposit needs = 1 Faraday = 96,500 coulombs. Therefore, 1.45 g of silver needs
= current x time
= 1295.6 C = 1.5 A x time (in sec.)
or time for which current is passed
The cathode reaction in copper sulphate cell is
2 x 96500 coulombs gives a deposit of 63.5 g of Cu.
Therefore, 1295.6 coulombs will deposit of
Similarly,
(cathode reaction in zinc sulphate cell)
Mass of zinc deposited
Calculate the standard cell potentials of galvanic cell in which the following reactions take place:
2Cr(s) + 3Cd2+ (aq) → 2Cr3+ (aq) + 3Cd
(Calculate the ΔrG° and equilibrium constant of the reaction.)
(Here n = 6 (as 6e are involved in overall cell reaction i.e.,
)
or
Calculate the standard cell potentials of galvanic cell in which the following reactions take place:
Fe2+ (aq) + Ag+ (aq) → Fe3+(aq) + Ag(s)
Calculate the ΔrG° and equilibrium constant of the reaction.
In the button cell widely used in watches and other devices the following reaction takes place:
Zn(s) + Ag2O(s) + H2O(l) → Zn2+(aq) + 2Ag(s) + 2OH– (aq)
Determine ΔrG° and E° for the reaction.
The resistance of a conductivity cell containing 0.001 M KCl solution at 298 K is 1500Ω. What is the cell constant if conductivity of 0.001 M KCl solution at 298 K is 0.146 x 10–3 s cm–1.
Conductivity of 0.00241 M acetic acid solution is 7.896 x 10–5 S cm-1. Calculate its molar conductivity in this solution. If Λ°m for acetic acid be 390.5 S cm2 mol–1, what would be its dissociation constant?
κ = 7.896 × 10−5 S m−1
Calculate the emf of the cell Zn/Zn2+ (0.1 M) || Cd2+ (0.01 M) | Cd at 298 k. (given)
E°Zn2+/Zn = – 0.76 V and E°Cd2+/Cd = – 0.40 V).
Zn/Zn2+(0.1 M) || Cd2+ (0.01) | Cd
Cu2+ + 2e– → Cu E° = + 0.34 V
Ag+ + 1e– → Ag E° = + 0.80 V
(i) Construct a galvanic cell using the above data.
(ii) For what concentration of Ag+ ions will the emf of the cell be zero at 25°C, if the concentration of Cu2+ is 0.01 M ? [log 3.919 = 0.593]
(i) Cu(s) | Cu2+ (aq) || Ag+ (aq) | Ag(s)
(ii) Cu(s) | Cu2+ (aq) || Ag+ (aq) | Ag(s)

Calculate the number of coulombs required for the oxidation of 1 mole of water to oxygen as per equation:
2H2O → 4H+ + O2 + 4e–
[Given: 1 F = 96,500 C mol–1]
2H2O → 4H+ + O2 + 4e–
2 moles of H2O require 4 x 96500 C
1 mole of H2O will need
The potential of a hydrogen electrode in a solution of unknown [H+] is 0.29 V at 298 k measured against a standard hydrogen electrode. Calculate the pH of the solution.
Since the potential of the hydrogen electrode measured against standard hydrogen electrode is a positive value, the electrode is acting as the anode. If the unknown [H+] be x, the Nernst equation takes the form
Since both electrodes are the same,
∴
or
Since
or
The measured resistance of a conductance cell containing 7.5 x 10–3 M solution of KCl at 25° C was 1005 ohms. Calculate (a) specific conductance (b) molar conductance of the solution cell constant = 1.25 cm–1.
Resistance = 1005ohm
cell constant =1.25 cm-1
thus
Specific conductance,
Estimate the minimum potential difference needed to reduce Al2O3 at 500° C. The free energy change for the decomposition reaction.
Since,
The emf (E°cell) of the cell reaction : 3Sn4+ + 2Cr → 3Sn2+ + 2Cr3+ is 0.89 V. Calculate ΔG° for the reaction. (F = 96,500 (mol–1 and VC ≡ J)
= – 6 x 0.89 V x 96500 C mol–1
= – 515310 J = – 515.310 kJ.
If E° for copper electrode is + 0.34 V how will you calculate its emf value when the solution in contact with it is 0.1 M in copper ions? How does emf for copper electrode change when concentration of Cu2+ ions in the solution is decreased?
Substituting the given values, we get
When concentration of Cu2+ ion in the solution decreases the emf of the electrode decreases. In this case it has decreased from 0.34 V to 0.3105 V.
Zinc rod is dipped in 0.1 M solution of ZnSO4. The salt is 95% dissociated at this dilution at 298 K. Calculate the electrode potential. [Given that: E°Zn2+/Zn = – 0.76 V.]
Concentration of
= 0.1 +
According to Nernst equation,
Calculate the resistance offered by 0.5 M CH3COOH solution when its molar conductivity. is 7.4 Ω–1 cm2 mol–1 and the cell constant is 0.037 cm–1.
cell constant 0.037cm-1
thus by using formula
and therefore
A 0.05 M NaOH solution offered a resistance of 31.6 Ω in a conductivity cell is 0.367 cm–1, find out the specific and molar conductance of the sodium hydroxide solution.
(i) Resistance (k) =
Conductance (C) =
=
Specific conductance (k)
= Conductance x cell constant
(ii) Molar conductance (C)
Molar conductance
Calculate the maximum possible electric work that can be obtained from the following cell under the standard conditions at 25°C:
The cell is
__________________________
___________________________
Here, n = 2
Therefore,
Zn | Zn2+ (α = 0.1 M) || Fe2+ (α = 0.01 M) | Fe. The emf of the above cell is 0.2905 V. What is the equilibrium constant for the cell reaction?
For cell
The cell reaction
On applying Nernst equation
or
or
or
At equilibrium
∴
or
or
or
When a certain conductivity cell was filled with 0.1 M KCl, it has a resistance of 85 Q at 25°C. When the same cell was filled with an aqueous solution of 0.052 M unknown electrolyte, the resistance was 96 Ω. Calculate the molar conductivity of the unknown electrolyte at this concentration. (Specific conductivity of 0.1 M KCl = 1.29 x 10–2 ohm–1cm–1).
Resistance of KCl solution,
R = 85
Cell constant = K x R
Resistance of unknown electrolyte solution,
Specific conductance
Concentration,
Molar conductance,
In a fuel cell, H2 and O2 react to produce electricity. In the process H2 gas is oxidised at the anode and O2 at cathode. If 67.2 litre of H2 at STP reacts in 15 minutes, what is the average current produced? If the entire current is used for electro-deposition of Cu from Cu2+, how many grams of copper are deposited?
At anode:
At cathode:
Therefore, moles of reacting
Therefore, equivalent of used =
Now,
Therefore,
or
Also Eq. of
Hence,
The following electrochemical cell has been set up
Pt(1) | Fe3+, Fe2+ (a = 1) || Ce4+, Ce3+ (a = 1) | Pt (2)
E°(Fe2+) = 0.77 V, and E°(Ce4+,Ce3+) = 1.61 V
If an ammeter is connected between the two platinum electrodes, predict the direction of flow of current. Will the current increase or decrease with time?
For the electrochemical cell
Pt(1) | Fe3+, Fe2+ (a = 1) || Ce4+, Ce3+ (a = 1) | Pt (2)
the cell regions are
Right-half cell: reduction
Left-half cell: oxidation
______________________________
Add
The net cell potential is
E° Cell = E° R – E° L = 1.61 V – 0.77 V = 0.84 V.
Since E°cell is positive, the cell reaction will be spontaneous.
The current in the external circuit will flow from Pt (1) (which serves as anode to Pt(2) which serves as cathode.
With the passage of time, Ecell will decrease and so is the current in the external circuit.
Conductivity of 0.00241 M acetic acid is 7.896 x 10–5 S cm–1. Calculate its molar conductivity, if Λ° for acetic acid is 390.5 S cm2 mol–1, what is its dissociation constant?
Concentration = 0.00241M
Conductivity =0.00241S Cm-1
thus by using the formula
we get
Write the Nernst equation and calculate the emf of the following cell at 298 K : Cu(s) | Cu2+ (0.130 M) || Ag+ (1.00 x 10–4 M) | Ag(s)
The standard reduction potential for the Zn2+ (aq) | Zn(s) half cell is 0.76 V. Write the reactions occurring at the electrodes when coupled with standard hydrogen electrode (SHE).
Zn acts as anode, and SHE acts as cathode
At anode: Zn(s) → Zn2+ (aq) + 2e–
At cathode: 2H+ (aq) + 2e– H2(g)
The net cell reaction is
Zn(s) + 2H+ (aq) → Zn2+ + H2(g)
Can a nickel spatula be used to stir the solution of CuSO4? Support your answer with reason. (E°Ni2+/Ni = – 0.25 V, E°Cu2+/Cu = + 0.34 V)
No.nickel spatula cannot be used to stir the solution of CuSO4
As E° Ni2+/Ni < E°cu2+/cu
Nickel has more tendency to lose electrons than copper. If a nickel spoon is used to CuSO4solution, it will displace Cu from CuSO4 solution.
Ni(s) + Cu2+ (aq) → Ni2+ (aq) + Cu(s)
Calculate the e.m.f. of the cell in which the reaction is
Calculate the cell e.m.f. at 25° C for the following cell:
Mg(s) | Mg2+ (0.01 M) || Sn2+ (0.1 M) | Sn (s)
[Given E°Mg2+/Mg = – 2.34 V, E°Sn2+/Sn = – 0.136V, 1 F = 96,500 C mol–1]
Calculate the maximum work that can be accomplished by the operation of this cell.
Mg(s) | Mg2+ (0.01 M) || Sn2+ (0.1 M) | Sn(s)
Mg(s) → Mg2+ (aq) + 2e–
(oxidation at anode)
Sn2+(aq) + 2e– → Sn(s)
(reduction at cathode)
Calculate the cell emf at 25° C for the following cell:
Ni(s) | Ni2+ (0.01 M) || Cu2+ (0.1 M) | Cu(s)
[Given E°Ni2+/Ni = – 0 25 V, E° Cu = + 0.34 V, 1 F = 96500]
Calculate the maximum work that can be accomplished by operation of this cell.
Ni(s) | Ni2+ (0.01 M) || Cu2+ (0.1 M) | Cu(s)
At anode Ni(s) → Ni2+ (aq) + 2e
At cathode Cu2+ + 2e → Cu(s)
Net cell reaction
The emf of a cell corresponding to the reaction
Zn(s) + 2H+ (aq) → Zn2+ (0.1 M) + H2(g) (1 atm) is 0.28 V at 15° C.Write the half cell reactions and calculate the pH of the solution at the hydrogen electrode.
Zn(s) + 2H+ (aq) → Zn2+ (0.1 M) + H2(g) (1 atm)
thus
Half call reaction will be
...(i)
Here
Therefore,
Similarly,
Now since
Using the standard electrode potentials given in the table 3.1(in NCERT), predict if the reaction between the following is feasible:
(i) Fe3+ (aq) and I– (aq)
(ii) Ag+ (aq) and Cu(s)
(iii) Fe3+ (aq) and Br–(aq)
(iv) Ag(s) and Fe3+(aq)
(v) Br2(aq) and Fe2+(aq)
From the table, standard electrode potents at 298 k are:
(E°Fe3+/VF = 0.77 V, E°I2/I– = 0.54 V)
(E°Ag+/Ag = E°Cu2+/Cu = 0.34)
(E°Fe3+/Fe= 0.77 V, E°Br2/Br- = 1.08 V)
(E°Ag+/Ag= 0.8 V E°Fe3+/Fe2+ = 0.77 V)
(E°Fe3+/Fe2+= 0.77 V,E°Br2/Br- = 1.08 V)
so that
(a)
In this reaction, Fe3+ is reduced to Fe2+ and I– is oxidised to I2. The cell giving above reaction will be
As E0 is positive, the reaction between Fe3+ (aq) and I– (aq) occurs as indicated by possible reaction given above.
(b)
Here, in this reaction, Ag+ is reduced to Ag (i.e., it should be cathode) and Cu(s) is oxidised to Cu2+(aq) (i.e., it should be anode).
The cell can be represented as
As E°cell is positive, the reaction between (Ag+ (aq) and Cu(s) occurs as indicated by possible reaction given above.
(c)
In this reaction Fe3+ is reduced to Fe2+ (i.e., Fe3/Fe2+ electrode should be cathode) and Br is oxidised to Br2 (i.e., Br2/Br– electrode should be anode.
The cell can be represented as:
As E°cell is negative, no reaction will occur between Fe3+ (aq) and Br–(aq).
(d)
Two half-cell reactions can be expressed as:
As E°cell is negative, no reaction occurs between Fe3+(aq) and Ag(s).
(e)
The two half-cell reactions are
As E°cell is positive, the reaction is feasible, i.e., reaction between Br2(aq) and Fe2+ (aq) occurs as indicated by possible reaction given above.
Write the Nernst equation and emf of the following cells at 298 k.
Mg(s) | Mg2+ (0.001 M) || Cu2+ (0.0001 M) | Cu(s)
For an electrochemical cell reaction
aA + bB → cC + dD
The Nernst’s equation for cell reaction is
The values of a, b, c, d and n can be obtained from the balanced cell reaction. (i) Anode reaction:
Cathode reaction:
Overall cell reaction:
Here, n= 2,
The Nernst equation for Ecell at 298 can be written as
Write the Nernst equation and emf of the following cells at 298 k.
Fe(s) | Fe2+ (0.001 M) || H+ (1M) | H2 (g) (1 bar) | Pt(s)
Cathode reaction:
Overall cell reaction:
The Nernst equation for Ecell at 298 k can be written as:
= 0.44 – 0.0295 (–3) = 0.44 – 0.0885 = 0.5285 V
Write the Nernst equation and emf of the following cells at 298 k.
Sn(s) | Sn2+ (0.050 M) || H+(0.020 M) H2(g) (1bar) Pt (s)
For the given cell Anode reaction:
Sn(s) → Sn2+ (aq) + 2e–
Cathode reaction:
2H+(aq) + 2e– → H2(g)
Overall cell reaction:
Sn(s) + 2H+ (aq) → Sn2+(aq) + H2(g)
Here, n = 2, E°cell = E°cathode – E°anode = 0 – (– 0.14 V) = + 0.14 V.
The Nernst equation for Ecell and 298 k can be written as:
Write the Nernst equation and emf of the following cells at 298 k.
Pt(s) | Br2 (l) | Br– (0.010 M) || H+ (0.030 M) | + H2(g) (1 bar) | Pt(s).
Cathode reaction:
Overall cell reaction:
Here, n = 2,
The Nernst equation for Ecell and 298 k can be written as:
The negative value of Ecell indicates the cell has been arranged in a reverse way, i.e., hydrogen electrode will act as anode and bromine electrode act as cathode. The cell should be represented as Pt | H2 (1 bar), H+ (0.03 M) || Br (0.01 M) | Br2(l), Pt
What is corrosion? What are the factors which affect corrosion?
Corrosion is the process of slowly eating away of the metal due to attack of the atmospheric gases on the surface of the metal resulting into the formation of compounds such as oxides, sulphides, carbonates, etc. The corrosion of iron is called rusting.
The phenomenon of corrosion involves the destruction of metal in which metal is generally converted into oxide. Common examples are rusting of rion, tarnishing of silver and deposition of green coating on copper and bronze.
Factors affecting corrosion:
(a) Presence of oxygen, sulphur etc. (elements which gain electrons).
(b) Presence of moisture.
(c) Presence of carbon dioxide.
CO2 is always present in natural water. Explain its effect (increases, stops or no effect) on rusting of iron.
Hydroxyl ion or bicarbonate ions attack the iron surface to form anodic regions in which iron loses electrons and pass on to ferrous sulphate which is further oxidised to ferric state by oxygen of the air. The released electrons move towards the cathode region where H+ions are converted into hydrogen gas.
Thus CO2 increases rusting because H2CO3 gives H+ which gain electrons to form H2 gas. The electrons are released by iron.
Rusting of iron is quicker in saline water than in ordinary water. Explain.
We can use aluminium in place of zinc for cathodic protection of rusting. Comment.
As the standard electrode potential of aluminium is more than that of zinc so Al is more anodic than Zn.
Thus we can better use aluminium in place of zinc for cathodic protection of rusting of iron.
At anode : Al → Al3+ + 3e–
At cathode : O2 + 2H2O + 4e– --->4OH–
How is cathodic protection of Iron different from its galvanisation?
Define conductivity and molar conductivity for the solution of an electrolyte. Discuss their variaion with concentration.
Molar Conductivity: Molar conductivity of a solution is defined as the conductance of all ions present in one mole of electrolyte in the solution. If M is the molar concentration in mol L–1, then.
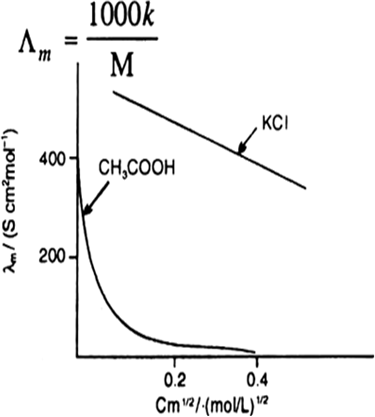
Fig: Molar conductivity versus C1/2 for acetic acid (week electrolyte) and potassium chloride (strong electrolyte in aqueous solutions)
The curve shown below gives the change in conductance against square root of concentrations. We observe that for strong electrolytes like KCl, the conductance does not change much with decrease in whereas in the case of weak electrolyte like acetic acid (CH3COOH) it increases much with decrease in
A current of 1.50 amp passed through an electrolytic cell containing AgNO3 solution with inert electrodes. The weight of Ag deposited was 1.50 g. How long did the current flow?
108 g of silver needs = 1 Faraday
= 96500 coulombs
1.50 g of silver needs =
But Q = current x time
1340.3 = 1.5A x time (in sec)
Time for which current is passed =
= 893.5 sec = 14.9 min
Write the reactions taking place at the anode and cathode in the above cell.
When AgNO3 is electrolysis in aqueous solution?
At cathode:
Give reactions taking place at the two electrodes if these are made up of Ag.
Electrolysis of AgNO3 using Ag electrodes
At cathode:
At anode:
The following chemical reaction is occuring in an electro-chemical cell
Mg(s) + 2Ag+(0.001 M) → Mg2+(0.10 M) + Ag(s)
The E° electrode values are
Mg2/Mg = – 2.36 V
Ag+/Ag = 0.81
V For the cell calculate/write:
(a) (i) E° value for the electrode 2Ag+/2Ag
(ii) Standard cell potential E°Cell
(b) Cell potential (E)cell
(c) (i) Symbolic representation of the above cell
(ii) Will the above cell reaction be spontaneous?
2Ag+(aq) + 2e– → 2Ag(s)
When a current of 0.75 A is passed through a CuSO4 solution for 25 min, .36 g of copper is deposited at the cathode. Calculate the atomic mass of copper.
Time = 25 min = 25 x 60 sec
Current = 0.75 A
Electricity passed = 25 x 60 x 0.75 = 1125 C of electricity deposit copper = 0.369 g. 2 x 96500 C
of electricity will deposit copper.
M= ZxIxT
Atomic mass of copper = 63.3 u.
Tarnished silver contains Ag2S. Can this tarnish removed by placing silver in an aluminium pan containing an inert electrolytic solution such as NaCl. The standard electrode potential for half reaction.
The conductivity of 0.001 M acetic acid is 4 x 10–5 s/cm. Calculate the dissociation constant of acetic acid, if λ°m for acetic acid is 390.5 s cm2 mol.
conductivity =4 x10-5 s/cm
In an electrolytic cell, the ___________ energy is generated at the expense of __________energy.
In a galvanic cell ________ energy is generated at the expense of _______ energy.
One coulomb is equal to - 96500 Faraday
- 6.28 x 1018 electrons
- 1 electron
- None of these
B.
6.28 x 1018 electronsHow is cell constant related to specific conductance of electrolyte?
The resistance of any conductor varies directly as its length (l) and inversely as its cross-sectional area (A), i.e.,
Mathematically
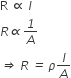
Where ρ is called the specific resistance.
l/A is known as cell constant.
If l = 1 cm and A = 1 cm2, then
R = ρ
The specific resistance is, thus defined as the resistance of one-centimetre cube of a conductor.
The reciprocal of specific resistance is termed the specific conductance or it is the conductance of one-centimetre cube of a conductor.
It is denoted by the symbol κ.
Thus,
![]()
Where (κ) kappa → the specific conductance
Specific conductance is also called conductivity.
Further,

or Specific conductance = Conductance × cell constant
In the case of electrolytic solutions, the specific conductance is defined as the conductance of a solution of definite dilution enclosed in a cell having two electrodes of unit area separated by one centimetre apart.
Unit of specific conductance: Ω-1 cm-1
Can we store aqueous copper sulphate solution in iron vessel?
It is not possible to store copper sulphate solution in iron vessel.since ,iron is more reactive than copper,it displaces copper from any if its solution.the reaction takes place as ,
Fe(s)+CuSO4(aq)..............>FeSO4(aq)+Cu(s)
Which type of electrolytes are used in salt bridge?
A salt bridge is a U-shaped device containing concentrated solution of an inert electrolyte like KCl, KNO3, etc.or a solidified solution of those electrolytes in agar-agar solution and gelatin. It connects the oxidation and reduction half-cells of a galvanic cell. The inert electrolytes present do not take part in redox reaction of the cell and dont react with the electrolyte that has been used.
As electrons leave one half of a galvanic cell and flow to the other, a difference in charge is built up. If no salt bridge were used, this increasing charge difference would eventually prevent further flow of electrons. The salt bridge solves these problems. It has two main functions:
1. To allow the flow of ions from one solution to another without mixing of the two solutions and completing the electrical circuit.
2. To maintain the electical neutrality of the solutions in the two half cells.
For a half-cell reaction: C6H5NO2 → C6H5NH2. What is the value of n in Nernst equation of electrode potential?
The value of n for electrode potential is 2.
What is the sign for G for a spontaneous cell reaction?
Free energy change G is measure of the spontaneity of a chemical reaction or process.
Where
-n =number of moles of electron
F= the quantity of electrical charge that is contained in 1mole of electrons.This is the Faraday constant i.e. 1F= 96500 Coloum/mole of electron
F and n are positive value. therefore as positive value of E (which indicte spontaneity )and thus negtive value for G (which also indicate spontaneity)
How can the standard oxidation potential of an electrode be raised up?
Standard Oxidation Potentials. The standard oxidation potential is much like the standard reduction potential. It is the tendency for a species to be oxidized at standard conditions.
it can be raised up by reducing hydrogen value in cell reaction.
How does free energy change related to emf of cell?
Gibbs Free Energy is defined as the thermodynamic potential that signifies the maximum or reversible work performed by a thermodynamic system at constant temperature and pressure. i.e.
What is the effect of decreasing concentration on the molar conductivity of weak electrolyte?
Weak electrolytes: When the concentration of weak electrolyte becomes very low, its degree of ionisation rises sharply. There is sharp increase in the number of ions in the solution. Hence, the molar conductivity of a weak electrolyte rises steeply at low concentration.
Fluorine cannot be prepared from fluorides by chemical oxidation.
Fluorine is the strongest oxidizing agent as it has highest E0 value. Therefore fluorine has the highest tendency to get reduced to F-. As a result, F- ion has the least tendency to get oxidized, hence fluorine cannot be prepared by chemical oxidation of fluorides.
Which factor makes secondary cells more acceptable as a source of power?
Secondary batteries are the rechargeable batteries. They have the advantage of being more cost-efficient over the long term, although individual batteries are more expensive. Generally, secondary batteries have a lower capacity and initial voltage, a flat discharge curve, higher self discharge rates and varying recharge life ratings.
Write Nernst equation for single electrode potential.
The concentration of all species involved in the species involved in the electrode reaction is unity.This need not be always true.
Nernst shows that for the electrode reaction:
the electrode potential at any concentration measured with respect to standard hydrogen electrode can be represented by:
but concentration of solid M is taken as unity as we have
R is gas constant (8.314 JK–1 mol–1),
F is Faraday constant (96487 C mol–1), T is temperature in kelvin and [Mn+] is the concentration of the species, Mn
Let us take a electrode reaction
The Nernst equation of this electrode
Instead of activity, we can take molar concentration.
For pure solid and liquid molar concentration is taken as unity.
Why is the equilibrium constant k related to only E°cell and not Ecell.
Nernst equation is given as:
Ecell =
Where Q reaction quotient
Ecell is the cell potential at the temperature of interest.
is the standard cell potential
R is the universal gas constant
T is the abolute temperture
F is the Farday constant
n i the number of electron
At equilibrium
Ecell =0 and Q=k
K isthe equilibrium constant
0=
How does the molar conductivity of KCl solution vary with increasing concentration?
Strong electrolytes: The molar conductivity of a strong electrolyte decreases slightly with the increase in concentration. This decrease is due to the increase in interionic attractions as a result of greater numbr of ions per unit volume. With dilution, the ions are far apart, inter ionic attractions become weaker and conductance increases
Give an example of fuel cell.
Fuel cell: A galvanic cell in which the reactants are continuously fed into the cell and the products are continuously removed is called a fuel cell.
The most important fuel cell is hydrogen oxygen fuel cell
Write the symbolic notation for standard hydrogen electrode and its potential.
A half-cell called standard hydrogen electrode
represented by Pt(s) l H2(g) l H+(aq), is assigned
a zero potential at all temperatures corresponding to the reaction
What is a galvanic cell? What type of reactions give rise to electric current in a galvanic cell and how?
A galvanic cell (left) transforms the energy released by a spontaneous redox reaction into electrical energy that can be used to perform work. The oxidative and reductive half-reactions usually occur in separate compartments that are connected by an external electrical circuit.
Oxidation half reaction:
Y ----> Y+ +e-
Reduction half reaction:
Z + e- ----> Z-
overall cell reaction
Y+Z ------> Y+ + Z- (G<0)
A second connection that allows ions to flow between the compartments (shown here as a vertical dashed line to represent a porous barrier) is necessary to maintain electrical neutrality. The potential difference between the electrodes (voltage) causes electrons to flow from the reductant to the oxidant through the external circuit, generating an electric current.
What information is conveyed by the cell rotation given below?
Cu(s) | Cu2+ (aq) (0.1 M) || Ag+ (aq) (0.2 M) | Ag(s)
In cell reaction :
Cu(s) +2Ag+(aq) ---->Cu2+(aq) +2Ag(s)
Half cell reaction
Cathode (reduction ):
2Ag+(aq) +2e- -----> 2Ag(s)
Anode (oxidation)
Cu(s) -----> Cu2+(aq) +2e-
In overall reaction of the cell, silver electrode act as cathode and copper electrode act as anode.
What is a salt bridge and what function does it save in a galvanic cell?
A salt bridge is a U-shaped device containing concentrated solution of an inert electrolyte like KCl, KNO3, etc.or a solidified solution of those electrolytes in agar-agar solution and gelatin. It connects the oxidation and reduction half-cells of a galvanic cell. The inert electrolytes present do not take part in redox reaction of the cell and dont react with the electrolyte that has been used.
As electrons leave one half of a galvanic cell and flow to the other, a difference in charge is built up. If no salt bridge were used, this increasing charge difference would eventually prevent further flow of electrons. The salt bridge solves these problems. It has two main functions:
1. To allow the flow of ions from one solution to another without mixing of the two solutions and completing the electrical circuit.
2. To maintain the electical neutrality of the solutions in the two half cells.
What is meant by ‘standard electrode potential’ on the hydrogen scale?
A half-cell called standard hydrogen electrode
represented by Pt(s)l H2(g)lH+(aq), is assigned
a zero potential at all temperatures corresponding to the reaction;
H+ (aq) + e- ---->1/2H2(g)
The standard hydrogen electrode consists of a platinum electrode coated with platinum black. The electrode is dipped in an acidic solution and pure hydrogen gas is bubbled through it.
and the concentration of hydrogen ion in the
solution is one molar.
How is an electrode potential measured?
The electrode potential measures the tendency of electrons to flow away from or towards a redox equilibrium. They are always measuredwith respect to the standard hydrogen electrode (which is assigned a value of zero volts).
Write a Nernst equation for the half-cell reaction: Mn+(aq) + ne– → M(s)
The concentration of all species involved in the species involved in the electrode reaction is unity.This need not be always true.
Nernst shows that for the electrode reaction:
the electrode potential at any concentration measured with respect to standard hydrogen electrode can be represented by:
but concentration of solid M is taken as unity as we have
R is gas constant (8.314 JK–1 mol–1),
F is Faraday constant (96487 C mol–1), T is temperature in kelvin and [Mn+] is the concentration of the species.
Write the Nernst equation for a galvanic cell corresponding to the reaction:
In Daniell cell, the electrode potential for any given concentration of
Cu2+ and Zn2+ ions, we write
For cathode:-
Electrolysis of molten NaCl gives sodium at cathode while aqueous NaCl gives H2 gas at cathode.
Molten NaCl dissociates to give Na+ and Cl-, Na+ then move towards cathode, picks up one electron and gets reduced to form Na.
Na+ +e- ---> Na
In aqueous NaCl, as , it is the hydrogen gas which liberates at cathode.
What is dry cell? Explain its working.
The cells from which electric energy is derived by irreversible chemical action are called primary cells. The primary cell is capable of providing an EMF when its constituent’s two electrodes and a suitable electrolyte are assembled together. The three main primary cells namely are the Daniel cell, the Leclanche cell, and the dry cell. None of these cells can be recharged electrically.
The dry cell consists of a zinc container that also acts as anode and the cathode is a carbon (graphite) rod surrounded by powdered manganese dioxide and carbon. The space between the electrodes is filled by a moist paste of ammonium chloride (NH4Cl) and zinc chloride (ZnCl2). The electrode reactions are complex, but they can be written approximately as follows :
Anode: Zn(s)----> Zn2+ + 2e–
Cathode: MnO2+ NH4+ + e–----> MnO(OH) + NH3
In the reaction at cathode, manganese is reduced from the + 4 oxidation state to the +3 state. Ammonia produced in the reaction forms a complex with Zn2+ to give [Zn (NH3)4]2+. The cell has a potential of nearly 1.5 V.
Draw curves to show how the molar conductance of strong electrolytes varies with dilutions.
Strong electrolytes: The molar conductivity of a strong electrolyte decreases slightly with the increase in concentration. This decrease is due to the increase in interionic attractions as a result of greater numbr of ions per unit volume. With dilution, the ions are far apart, inter ionic attractions become weaker and conductance increases.
How the Kohlrausch’s law is used to determine the degree of ionization of weak electrolyte?
Kohlrausch’s law of independent migration of ions states molar conductivity of an electrolyte at infinite dilution can be expressed as the sum of the contribution of individual ions. If molar conductivity of cations and anions are represented by λ∞+ and λ∞– respectively.
where v+ and v– are number of cations and anions per formula of electrolyte e.g.,
Λ∞ CaCl2 = λ∞ (Ca2+) + 2 λ∞ (CI–)
Λ = KCl = λ∞ (K+) + λ∞ (CI–)
Uses 1. It is used to find molar conductivity of weak electrolyte at infinite dilution which
cannot be obtained by extrapolation.
2. It is used to calculate degree of dissociation of weak electrolyte at a particular concentration.
Degree of dissociation
where Λm is molar conductivity of weak electrolyte at a particular concentration and Λemis molar conductivity of weak electrolyte at infinite dilution.
For the cell Zn/Zn2+ (aq) || Cu2+(aq) | Cu, derive the relation between E°cell and Kc at 298 k.
Gibbs energy of the reaction given by:
= – nFE(cell)
thus the reaction
Zn(s) + Cu2+(aq)---> Zn2+(aq) + Cu(s)
= – 2FE(cell)
but when we write the reaction
2 Zn (s) + 2 Cu2+----->2 Zn2+(aq) + 2Cu(s)
= – 4FE(cell)
If the concentration of all the reacting species is unity, then
E(cell) =
and we have
= – nF
Thus, from the measurement of we can obtain an important thermodynamic quantity, , standard Gibbs energy of the reaction.
From the latter we can calculate equilibrium constant by the equation:
= –RT ln Kc
What do you mean by a secondary cell? Discuss the function of lead storage battery.
A secondary cell after use can be recharged by passing current through it in the opposite direction so that it can be used again. A good secondary cell can undergo a large number of discharging and charging cycles. The most important secondary cell is the lead storage battery commonly used in automobiles and invertors.
It consists of a lead anode and a grid of lead packed with lead dioxide (PbO2 ) as cathode. A 38% solution of sulphuric acid is used as an electrolyte.
The cell reactions when the battery is in use are given below:
Anode: Pb(s) + SO42–(aq)----> PbSO4(s) + 2e–
Cathode: PbO2(s) + SO42–(aq) + 4H+(aq) + 2e– ----> PbSO4 (s) + 2H2O (l )
i.e., overall cell reaction consisting of cathode and anode reactions is:
Pb(s) + PbO2(s) + 2H2SO4(aq)---> 2PbSO4(s) + 2H2O(l)
On charging the battery the reaction is reversed and PbSO4(s) on anode and cathode is converted into Pb and PbO2, respectively.
Define conductivity and molar conductivity for the solution of an electrolyte.

On the basis of the standard electrode potential values stated for acid solution. Predict whether Ti4+ species may be used to oxidise FeII to FeIII.
Reaction: E°/V TiIV + e– → Ti3+ + 0.01
Fe3+ + e– → Fe2+ + 0.77
Ti4+ would not oxidize Fe+2 . since the standard electrode potential of iron is high as compare to the titanuim standard electrode potential.
What are fuel cells? Write the electrode reactions of a fuel cell which uses the reaction of hydrogen with oxygen.

Hydrogen-oxygen fuel cell: The cell consists of three compartments separated from one another by porous electrode. The hydrogen gas is fed into one compartment and the oxygen gas is fed into another compartment. These gases then diffuse slowly through the electrodes and react with an electrolyte that is in the central compartment. The electrodes are made of a conducting material, such as graphite, with a sprinkling of platinum to act as a catalyst, and the electrolyte is an aqueous solution of a base. The reactions are
Anode: 2H2 (g) + 4OH–(aq)-----> 4H2O(l) + 4e–
Overall reaction being:
2H2(g) + O2(g) ----->2H2O(l )
(ii) The only product in the reaction of fuel cell is water which can be removed and the astronauts of a spacecraft can drink it.
Predict the products of electrolysis obtained at the electrodes in each case when the electrodes used are of platinum.
(i) An aqueous solution of AgNO3.
(ii) An aqueous solution of H2SO4.
Reaction in solution
Define the following terms: (i) Cathodic protection, (ii) Electrochemical series, (iii) Cell constant, (iv) Equivalent conductivity, (v) Strong and weak electrolytes.
(i) Cathodic protection (CP) is a technique used to control the corrosion of a metal surface by making it the cathode of an electrochemical cell. A simple method of protection connects the metal to be protected to a more easily corroded "sacrificial metal" to act as the anode.
for example zinc is used to pervent iron
Zinc is more electro-positive than iron. Therefore, as long as zinc is there on the iron pipe, zinc acts as anode and the iron as cathode. As a result, rusting of iron is prevented.
(ii)Electrochemical series is a series of chemical elements arranged in order of their standard electrode potentials. The hydrogen electrode. H+(aq) + e- →← 1/2H2(g) is taken as having zero electrode potential. An electrode potential is, by definition, a reduction potential
(iii)The quanitty 1/A is called cell constant denoted by the symbol. G*. It depends on the distance between the electrodes and their area of cross -section and has the dimension of length-1 and can be calculated if l and A
G* =l/A =Rk
(iv) A strong electrolyte is a solute that completely, or almost completely, ionizes or dissociates in a solution. While the specificconductance of a solution increases with concentration, the equivalent conductance decreases as the concentration increases. unit of equivalent conductance
(v)electrolytes :A substance that when dissolved in water produced a solution that can conduct electric current.
there are two electrolytes
1. strong
2.weak
strong Electrolytes conduct current very efficiently.Completely ionized or dissociate when dissolved in water
a. Soluble Ionic compounds
b. Strong acids (HNO3(aq), H2SO4(aq), HCl(aq))
HNO3--> H+ + NO3- (100% ionization)
c. Strong bases (KOH and NaOH)
KOH -->K+ +OH - (100% dissociation)
Weak electrolytes conduct only a small current
Slightly ionized in solution
a. Weak acids (organic acids-->acetic, citric, butyric,malic, etc.)
HC2H3O2 <==> H+ + C2H3O2-
b. Weak bases (ammonia)
NH3 + H2O <==> NH4+ + OH-
Explain the working of galvanic cell. How does the electrochemical cell differ from electrolytic cell?
A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through.
If we connect the zinc and copper by means of a metallic conductor, the excess electrons that remain when Zn2+ ions emerge from the zinc in the left cell would be able to flow through the external circuit and into the right electrode, where they could be delivered to the Cu2+ ions which become "discharged", that is, converted into Cu atoms at the surface of the copper electrode. The net reaction is the oxidation of zinc by copper(II) ions:
but this time, the oxidation and reduction steps (half reactions) take place in separate locations:
left electrode : Zn----> Zn2+ +2e-right electrde: Cu2+ +2e- ----> Cu

Diffrance between galvanic cell and electrotic cell
| galvanic cell | Electrolytic cell |
|---|---|
| A Galvanic cell converts chemical energy into electrical energy. | An electrolytic cell converts electrical energy into chemical energy. |
| Here, the redox reaction is spontaneous and is responsible for the production of electrical energy. | The redox reaction is not spontaneous and electrical energy has to be supplied to initiate the reaction. |
| The two half-cells are set up in different containers, being connected through the salt bridge or porous partition. | Both the electrodes are placed in a same container in the solution of molten electrolyte. |
| Here the anode is negative and cathode is the positive electrode. The reaction at the anode is oxidation and that at the cathode is reduction. | Here, the anode is positive and cathode is the negative electrode. The reaction at the anode is oxidation and that at the cathode is reduction. |
| The electrons are supplied by the species getting oxidized. They move from anode to the cathode in the external circuit. | The external battery supplies the electrons. They enter through the cathode and come out through the anode. |
What is normal hydrogen electrode? Discuss its uses.
Definition: The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials.
The standard is determined by the potential of a platinum electrode in the redox half reaction
2 H+(aq) + 2 e- → H2(g) at 25 °C.
The standard hydrogen electrode is often abbreviated SHE.
Also Known As: normal hydrogen electrode or NHE
What is corrosion? What are the factors which affect corrosion?
Corrosion is the process of slowly eating away of the metal due to attack of the atmospheric gases on the surface of the metal resulting into the formation of compounds such as oxides, sulphides, carbonates, etc.
The rusting of iron, tarnishing of silver, development of green coating on copper and bronze are some of the examples of corrosion.
The main factors which affect corrosion are
Presence of water and the electrolytes present in it.
1. More the reactivity of metal, the more will be the possibility of the metal getting corroded.
2. The impurities help in setting up voltaic cells, which increase the speed of corrosion
3. Presence of electrolytes in water also increases the rate of corrosion
4. Presence of CO2 in natural water increase rusting of iron.
5. When the iron surface is coated with layers of metals more active than iron, then the rate of corrosion is retarded.
6. A rise in temperature (with in a reasonable limit) increases the rate of corrosion.
Distinguish between: (a) Electrolytes and non-electrolytes, (b) Reduction potential and oxidation potential (c) Primary cells and secondary cells, (d) Specific conductivity and molar conductivity.
a)
|
electrolytes |
nonelectrolyte |
|
An electrolyte dissociates in solution and thus produce ion.
|
A nonelectrolyte does not dissociate at all in solution and therefore does not produce any ions. |
|
Electrolytes are ionic substance that dissolve in water |
Nonelectrolytes are typically polar covalent substances that do dissolve in water as molecules instead of ions. |
C)
|
Primary cell |
Secondary |
|
Lower initial cost. |
Higher Initial Cost |
|
Higher life-cycle cost ($/kWh). |
Lower life-cycle cost ($/kWh) if charging in convenient and inexpensive |
|
Disposable. |
Regular maintenance required. |
|
Typically lighter and smaller thus traditionally more suited for portable applications. |
Traditionally less suited for portable applications, although recent advances in Lithium battery technology have lead to the development of smaller/lighter secondary batteries. |
d)
|
Molar conductivity |
Specific conductivity |
|
Molar Conductivity of a solution at a given concentration is the conductance of the volume V of solution containing one mole of electrolyte kept between two electrodes with area of cross section A and distance of unit length. Therefore, Distance is unit so l = 1 Volume = area of base × length So V = A × 1 = A Λm =κA/l Λm = κV
|
Conductivity of a solution is equal to the conductance of a solution of 1 cm length and cross section area of 1 square cm. it may also be define as the conductance of ine centimeter cube of the conductor . It is represented by the symbol Kappa (κ). mathematically we can write κ = 1/ p here ρ is resistivity the unit of K is ohm –1 cm –1 or S cm–1 The conductivity, κ, of an electrolytic solution depends on the concentration of the electrolyte, nature of solvent and temperature.
|
The conductivity of 0.01 M solution of acetic acid at 25°C is 1.63 x 10–4 s cm–1. Given:
Λ°m (HCl) = 426 s cm2 mol–1, Δ°m (Na AC) = 91.5 cm2 mol–1
Λ°m (NaCl) = 126.5 cm2 mol–1 Calculate:
(a) the molar conductivity of acetic acid
(b) the degree of dissociation of acetic acid.
(c) the dissociation constant.
(d) the pH of 0.01 M solution of acetic acid.
a) The molar conductivity of acetic acid given by;
Calculate the equilibrium constant for the reaction:
Zn + Cd2+ (aq) → Zn2+ (aq) + Cd (E°cell = 0.36 V)
we have given that
Ecell0 =0.36V
thus equilibrium constant is
n=2
k =1.596 x 1012
Calculate the pH of the following half reactions: Pt. H2 (1 atom) / HCl, E = 0.25 V.
H2 ----> 2H++2e-
Ans. pH = 4.237
Electrolytic conductivity of 0.20 mol L–1 solution of KCl at 298 k is 2.48 x 10–2 ohm–1cm–1. Calculate its molar conductivity.
we have given that
electrolyitc conductivity =0.20mol/L
conductivity = 2.48 x 10-2 ohm-1cm-1
thus apply the formula
here M is molar conductivity.
Ans. 124 ohm–1 cm2 mol–1
Calculate the molar conductivity at infinite dilution of acetic acid from the following data:
Λ∞m (HCl) = 426 ohm–1 cm2 mol–1, Λ∞m CH3COONa = 91 ohm–1 cm2 mol–1 and Λ∞m(NaCl) = 126 ohm–1 cm2 mol–1.
Acetic acid is weak electrolyte than HCl and NaCl which is strong electrolyte.
Acoording to kohlransch's law
=91-126+426 =391
Ans. 391 ohm–1 cm2 mol-1
Calculate the equilibrium constant of reaction at 25°C:
Ni(s) + Cu2+ (aq) → Cu(s) + Ni2+ (aq)
Given : E°Ni2+/Ni = -0.25 V, E°Cu2+/Cu = + 0.34 V, R = 8.314 J K–1 mol–1, F = 96500 C mol–1.
We have given
E°Ni2+/Ni = -0.25 V
E°Cu2+/Cu = + 0.34 V,
Ecell0 =
= 0.34-(-0.25)
= 0.34+0.25 =0.59
R = 8.314 J K–1 mol–1
F = 96500 C mol–1.
T=25celcius =273+25 =298 Kelvin
n= 2
= Antilog[19.956]
Ans. 9.07 x 1019
Calculate the standard free energy change for the reaction occuring in the cell:
Zn(s) | Zn2+ (1 M)|| Cu2+ (1M) | Cu(s)
[Given E°Zn2+/Zn = – 0.076 V, E°Cu2+/Cu = + 0.34 V, F = 96500 C mol–1]
= – 0.076 V
= + 0.34 V
F = 96500 C mol–1
from the reaction
n=2
= -
=0.34 -(-0.076)
= 0.416
We know that
= -2 x 96500 x 0.416
=–802.88 kJ mol–1
Consider the cell Zn/Zn2+ (aq) (1.0 M) || Cu2+ (aq) (0.1 M) | Cu The standard reaction potentials are + 0.35 V for 2e– + Cu2+ (aq) → Cu and – 0.763 V for 2e– + Zn2+ (aq) → Zn
(i) Write down the cell reaction.
(ii) Calculate the emf of the cell.
(iii) Is the cell reaction spontaneous or not?
(I)Cell Reaction:
Oxidation: Zn----> Zn2+ +2e-
Reduction: Cu2+ +2e------> Cu
Therefore overall reaction
Zn +Cu2+ ------> Zn2+ +Cu
(ii) =+0.35-(-0.763)
=1.113 volts
(iii) Since EMF of cell is positive, it is a spontaneous reaction.
The standard reaction potential for Cu2+/Cu is + 0.34 V. Calculate the reduction potential at pH = 14 for the above couple. Ksp of Cu(OH)2 is 1.0 x 10–19.
reduction potential is given by:
From the given data PH =14 and KsP (Cu(OH2) =1.0 x 10-19
we get
H+ =10-14 M thus,
[OH]- =
ans -0.22V
The standard reduction potential of the reaction at 25°C
2H2O + 2e– H2(g) + 2OH– is – 0.8277 V
Calculate equilibrium constant for the reaction
at
Consider the given reaction as the net cell reaction
two half reaction :
Oxidation:
H2O +1/2H2 ----> H3O+ +e- Ecell0 =0
reduction:
H2O +e- -----> 1/2H2(g) + OH- Ecell0 = -0.8277V
It is evident from the cell reaction that it involves the transfer of one electron so that n= 1
The resistance of a conductivity cell containing 0.01 M KCl solution at 298 K is 1500 Ω. What is the cell constant if conductivity of 0.001 M KCl solution at 298 K. is 1.46 x 10–6 s cm–1.
We ave given that
R = 1500 Ω
K = 1.46 x 10–4 Ω–1 cm–1
Cell constant = K x R
= 1.46 x 10–4 x 1500 = 0.219
The conductivity of 0.1 M KCl solution at 298 K is 0.0129 s cm–1. The resistance of this solution in a conductivity cell is found to be 58 ohms. What is the cell constant of the cell? The 0.1 M AgNO3 solution at 298 K in the same conductivity cell offered a resistance of 60.5 ohms. What is the conductivity of 0.1 M AgNO3 solution?
KKCl = 0.0129 s cm–1
RKCl = 58 Ω
cell constant= k x R
cell constant= 0.0129 x 58
Cell constant = 0.7482 cm–1
As AgNO3 is also in the same conductivity cell, the cell constant remains same. Therefore,
Conductivity of
Resistance of a conductivity cell filled with 0.1 M KCl solution is 100 £2. If the resistance of the same cell when filled with 0.02 M KCl solution is 520 Ω. Calculate the conductivity and molar conductivity of 0.02 M KC1 solution. The conductivity of 0.1 M KCl at 298 K is 0.0129 s cm–1.
Cell constant = K x R
=
The conductivity of 0.00241 M acetic acid is 7.896 x 10–5 s cm–1. Calculate its molar conductivity and if Λ°m for acetic acid is 390.5 s cm2 mol–1, what is its dissociation constant?
M = 0.00241 M
K = 7.896 x 10-5 s cm-1
Λ°m for NaCl, HCl and CH3COONa are 126.4, 425.9 and 91.0 s cm2 mol–1respectively. Calculate Λ° for HAC.
Λ° (HAC) = λ°(H+) + λ°(AC–)
= λ° (H+) + λ°(Cl–) + λ° (AC–) + λ° (Na+) – λ°(Cl–) – λ°(Na+)
= Λ° (HCl) + Λ° (NaAc) – Λ° (NaCl)
= (425.9 + 91.0 – 126.4) s cm2 mol–1 = 390.5 s cm2 mol–1
The specific conductance of a saturated solution of AgCl in water is 1.826 x 10–6ohm–1 cm–1 at 25°C. Calculate its solubility in water at 25°C. [Given Λ∞m (Ag+) = 61.92 ohm–1 cm2 mol–1 and Λ∞m (CI– ) = 76.34 ohm–1 cm2 mol–1]
Calculate the molar conductance at infinite dilution of ethanoic acid from the following data:
Λ°m (HCl) = 425.9 s cm2 mol–1
Λ°m (CH3COONa) = 91.0s cm2 mol–1
Λ°m (NaCl) = 126.4 s cm2 mol–1
Λ°m (CH3COOH) = λ°H+ + λ°CH3coo–
= Λ°m(HCl) = λ°m (CH3COONa) – λ°NaCl
= 425.9 + 91.0 –126.4
= 390.5 s cm2 mol–1.
Draw curves to show how the molar conductance of strong electrolytes varies with dilution.
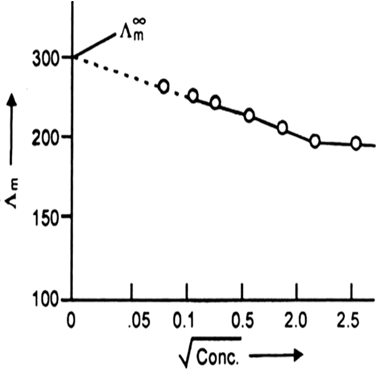
The molar conductivity at infinite dilution (Λ∞m) can be obtained by extrapolating the above graph of strong electrolytes to zero concentration.
Kohlrausch Law of Independent migration of ions: The law states that limiting molar conductivity of an electrolyte can be represented as the sum of the individual contributions of the anion and cation of the electrolyte. Thus, if λ°Na+ and λCl– are molar limiting conductivity of the sodium and chloride ions respectively, then the limiting molar conductivity for sodium chloride is given by the equation:
Λ°NaCl = λ°Na + λCl–
In general, if an electrolyte on dissociation gives v+ cations and v– anions then its limiting molar conductivity is given by
Λ° = v + λ°+ + V– λ°–
Here, λ°+ and λ°– are the limiting molar conductivities of the cation and anion respectively.
Kohlrausch’s law helps us to calculate
(i) Determination of molar conductivities of weak electrolytes at infinite dilution.
(ii) Determination of the degree of dissociation of electrolytes.
Degree of dissociation: It is ratio of molar conductivity at a specific countraction ‘C’ to the molar conductivity at infinite dilution. It is denoted by α.
i.e.,
The molar conductivity of KCl solutions at different concentration at 298 K are given below:
|
C/mol L–1 |
Λ/s cm2 mol–1 |
|
0.000198 0.000309 0.000521 0.000989 |
148.61 148.29 147.81 147.09 |
|
C1/2 /(mol L–1)1/2 |
A/s cm2 mol–1 |
|
0.01407 0.01758 0.02283 0.03145 |
148.61 148.29 147.81 147.09 |

A plot of A(l /-axis) and c1/2 (x-axis) is shown in the fig. It is nearly a straight line. From the intercept (C1/2 = 0) we find that Λ° = 149.8 s cm2 mol–1 and Slope = A = 87.46s cm2 mol–1/(mol / L–1)1/2.
How long will it take to deposit electrolytically 127 gm of copper on the cathode of a copper voltameter by a current of 50A? Given ECE of copper = 0.0003294 g/c.
We have given that
m = 127 gm,
I = 50 A
Z = 0.0003294 g/c
t = ?
By Faraday's first law,
m = ZIt
or
t = 77118 sec.
An electric current of 0.5 ampere was passed through acidulated water for one hour. Calculate the volume of hydrogen at STP produced. 1 coulomb of electricity deposits 0.00001 gm of hydrogen.
We have given that
current = 0.5 ampere
weight of H2 (W) = ?
t = 1 hour = 3600 sec,
Z = 0.00001.
W = Z x c x t
= 0.00001 x 0.5 x 3600 g = 0.018 g
2g or H2 at STP occupies = 22.4 litres
0.018 g of H2 at STP occupies
Hence, Volume of H2 produced at STP = 0.2016 litres.
How much charge is required for the following reduction of
(i) 1 mol of Al3+ to Al
(ii) 1 mol of Cu2+ to Cu
(iii) 1 mol of MnO4– to Mn2+
(i) Al3+ + 3e– → Al
1 mol Al3+ for reduction requires 3 mol e– or 3F electricity
1F = 96500C
∴ 3F = 3 x 96500 = 289500 C
(ii) Reduction of 1 mol Cu2+ to Cu requires 2 mol electrons
2 mol electrons = 2F = 2 x 96500 = 193000 C
(iii) In the reduction of 1 mol MnO–4 to Mn2+, there is net gain of 5e–
MnO4 + 5e– + 8H+ → Mn2+ + 4H2O
5F = 5 x 96500 = 482500 C
A solution of CuSO4 is electrolysed for 10 minutes with a current of 1.5 amperes. What is the mass of copper deposited at the cathode? (at mass Cu = 63.5).
The cathode reaction is Cu2+ + 2e– → Cu
I = 1.5 amperes, t = 10 x 60 = 600 s
∴ Q = It = 1.5 x 600 = 900 C
The reaction states that 2 x 96500 C are required to deposit 63.5 g Cu at cathode
900 C will deposit
A solution of CuSO4 is electrolysed for 10 minutes with a current of 1.5 amperes. What is the mass of copper deposited at the cathode?
t = 600 s
Charge = current x time
= 1.5 A x 600s = 900 C
According to the reaction : Cu2+(aq) + 2e– → Cu(s)
We require 2F or 2 x 96487 C to deposit 1 mol or 63 g of Cu
For 900 C, the mass of Cu deposited =
Represent the cell in which the following reaction takes place:
Mg(s) + 2Ag+ (0.0001 M) → Mg2+(0.130 m) + 2Ag(s)
Calculate its E(cell), if E°cell = 3.17 V.
Mg(s) | Mg2+(aq) (0.130 M) || Ag+(aq) (0.0001 M) | Ag(s)
The Nernst equation is
A Cell is prepared by dipping a copper rod in 0.01 M copper sulphate solution, and zinc rod in 0.02 M ZnSO4 solution. The standard reduction potentials of copper and zinc are + 0.34 V and – 0.76 V respectively.
(a) What will be the cell reaction?
(b) How will the cell be represented?
(c) What will be the emf. of the cell?
(a) As E°Zn2+/Zn < E°Cu2+/Cu . Zinc electrode acts as anode and copper electrode acts as a cathode. The cell reaction is
Zn(s) + Cu2+(aq) (0.01 M) → Zn2+(aq) (0.02 M) + Cu(s)
(b) The cell is represented as
Zn(s) | Zn2+(aq) (0.02 M) || Cu2+(aq) (0.01 M) + Cu(s)
(c) The emf of the cell can be calculated by using Nernst equation
Calculate the standard electrode potential of the Mg2+ / Mg electrode for a cell in which the cell reaction is
Mg(s) + 2Ag+(aq) → Mg2+(aq) + 2Ag(s)
Given that, [Mg2+] = 0.1 M, [Ag+] = 0.01 M
E°Ag/Ag = + 0.80, Ecell = + 2.90 V
The cell is
Mg(s) | Mg2+ (aq) (0.1 M) || Ag+ (aq) (0.01 M) | Ag(s)
and the Ecell is given by
But
Write the Nernst equation and calculate the emf of the following cell at 298 K:
Pt(s) | Br2 (l) Br– (0.01M) || H+ (0.03 M) | H2(g) (1 bar) | Pt(s)
Given E°Br2/Br– = + 1.08 V
Br2 / Br–
The Nernst equation is
Calculate the equilibrium constant of the reaction
given : E0 =0.46 temperture 250 C
lnk =2 x96500 x0.46/0.059
Determine the equilibrium constant of the reaction at 298 K.
From the obtained value of the equilibrium constant, predict whether Sn2+ ions can reduce Fe3+ to Fe2+ quantitatively or not.
The cell is
and
and n = 2
∴
As K is very high, the reaction is favoured in the forward direction, so, Sn2+ can easily reduce Fe3+ ion to Fe2+ ion.
Calculate the equilibrium constant for the reaction at 298 K
Given:
Equilibrium constant,
or
Calculate the equilibrium constant Kc for the reaction at 298 K.
It is clear from the given equation that electrons involved in the reaction (n) = 6
or
or
or
Calculate the equilibrium constant for the cell reaction:
(Given )
E0 = 0.16
Calculate the potential (emf.) of the cell
Cd | Cd2+ (0.10 M) || H+(0.20 M) | Pt, H2 (0.5 atm)
(Given : E° Cd2+ / Cd = – 0.403 V, R = 8.314 K–1 K–1 mol–1, F = 96500 C mol–1)
Equilibrium Constant from Nernst Equation: If the Daniell cell is short circuited, then we note that the reacton.
Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
takes place and as time passes, the concentration of Zn2+ keeps on increasing while the concentration of Cu2+ keeps decreasing. At the same time, voltage of the cell as read on the voltmeter keeps on decreasing. After some time, we shall note that there is no change in the concentration of Cu2+ and Zn2+ ions and at the same time, voltmeter gives zero reading. This indicates that equilibrium has been attained. In this situation the Nernst equation may be written as
But at equilibrium [Zn2+] / [Cu2+] = K
and the above equation can be written as
(E° = 1.1 V)
K = 2 x 1037 at 298
In general E°cell = 2.303 RT / nF x log K
Following reactions occur at cathode during the electrolysis of aqueous silver chloride solution:
Ag+ (aq) + e- → Ag(s) E° = +0.80 V
H+ (aq) + e- → 1/2 H2 (g) E° = 0.00 V
On the basis of their standard reduction electrode potential (E°) values, which reaction is feasible at the cathode and why?
We have given:
Ag+ (aq) + e- → Ag(s) E° = +0.80 V
H+ (aq) + e-→ 1/2 H2 (g) E° = 0.00 V
The relationship between the standard free energy change and emf of a cell reaction is given by
∆ G = – nFE(cell)
Thus, the more positive the standard reduction potential of a reaction, the more negative is the standard free energy change associated with the process and, consequently, the higher is the feasibility of the reaction.
Since E 0 Ag+/Ag has a greater positive value than E0 H+ /H, the reaction which is feasible at the cathode is given by
Ag+ (aq) + e- → Ag(s)
Define limiting molar conductivity. Why conductivity of an electrolyte solution decreases with the decrease in concentration?
The limiting molar conductivity of an electrolyte is defined as its molar conductivity when the concentration of the electrolyte in the solution approaches zero.
The conductivity of an electrolyte solution is the conductance of ions present in a unit volume of the solution. The number of ions (responsible for carrying current) decreases when the solution is diluted or the concentration is decreased. As a result, the conductivity of an electrolyte solution decreases with the decrease in concentration.
Calculate emf of the following cell at 25 °C:
Fe | Fe2+(0.001 M) || H+ (0.01 M) | H2 (g) (1 bar) | Pt(s)
E°(Fe2+ | Fe) = –0.44 V E°(H+ | H2 ) = 0.00 V
For the given cell representation, the cell reaction will be Fe(s) + 2H+(aq) → Fe2+(aq) + H2(g)
The standard emf of the cell will be
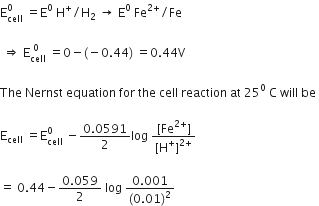
=0.44-0.02955(log10)
=0.44-0.02955(1)
=0.41045V 0.41V
The Nernst equation for the cell reaction at 25 º C will be
=0.44-0.02955(log10)
=0.44-0.02955(1)
=0.41045V 0.41V
Define the following terms:
(i) Limiting molar conductivity
(ii) Fuel cell
(i) When the concentration of an electrolyte approaches zero, then its molar conductivity is known as limiting molar conductivity.
(ii) Fuel cells are the galvanic cells in which the energy of combustion of the fuels likes hydrogen, methanol. etc is directly converted into electrical energy.
The resistance of a conductivity cell filled with 0.1 mol L-1 KCl solution is 100 . If the resistance of the same cell when filled with 0.02 mol L-1 KCl solution is 520 ![]() , calculate the conductivity and molar conductivity of 0.02 mol L-1 KCl solution. The conductivity of 0.1 mol L-1 KCl solution is 1.29x 10-2
, calculate the conductivity and molar conductivity of 0.02 mol L-1 KCl solution. The conductivity of 0.1 mol L-1 KCl solution is 1.29x 10-2 ![]() -1cm-1
-1cm-1
Given that:
Concentration of the KCl solution = 0.1 mol L-1
Resistance of cell filled with 0.1 mol L-1 KCl solution = 100 ohm
Cell constant = G* = conductivity x resistance
1.29x10-2 ohm-1 cm-1 x 100 ohm = 1.29 cm-1 = 129 m-1
Cell constant for a particular conductivity cell is a constant.
Conductivity of 0.02 mol L-1 KCl solution = =0.248 Sm-1
Concentration = 0.02 mol-1
= 1000x 0.02 mol m-3 = 20 mol m-3
Now,
Molar conductivity =
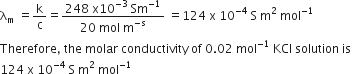
State Faraday's first law of electrolysis. How much charge in terms of Faraday is required for the reduction of 1 mol of Cu2+ to Cu.
Faraday's first law of electrolysis states that 'the amount of chemical reaction which occurs at any electrode during electrolysis by a current is proportional to the quantity of electricity passed through the electrolytic solution or melt'.
The reduction of one mol of Cu2+ to Cu can be represented as:
Cu2++ 2e-----> Cu
Since, in this reaction, there are two moles of electrons involved, so the amount of charge required is 2F.
Calculate emf of the following cell at 298 K: Mg(s) | Mg2+(0.1 M) || Cu2+ (0.01) | Cu(s)
[Given E0 cell = +2.71 V, 1 F = 96500 C mol-1]
The cell reaction can be represented as:
Mg(s) + Cu2+(aq.) ---> Mg+(aq.) + Cu(s)
Given:
![]() =+2.71 V
=+2.71 V
T = 298 K
According to the Nernst equation:
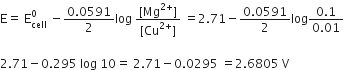
=2.71-0.0295 log 10 = 2.71-0.0295
=2.6805 V
a) What type of a battery is the lead storage battery? Write the anode and the cathode reactions and the overall reaction occurring in a lead storage battery when current is drawn from it.
(b) In the button cell, widely used in watches, the following reaction takes place
![]()
Determine E° and G° for the reaction ![]()
A lead storage battery has a secondary cell. Thus, it can be recharged by passing direct current through it. Therefore, it can be reused. It is used in automobiles.
In a lead storage cell, the anode is made of spongy lead and the cathode is a grid of lead packed with lead dioxide. The electrolyte used is H2SO4.

(a) Define molar conductivity of a solution and explain how molar conductivity changes with a change in concentration of solution for a weak and a strong electrolyte.
(b) The resistance of a conductivity cell containing 0.001 M KCl solution at 298 K is ![]() 1500 . What is the cell constant if the conductivity of 0.001 M KCl solution at 298 K is 0.146x10-3 S cm-1?
1500 . What is the cell constant if the conductivity of 0.001 M KCl solution at 298 K is 0.146x10-3 S cm-1?
Molar conductivity of a solution at a given concentration is the conductance of volume V of a solution containing 1 mole of the electrolyte kept between two electrodes with the area of cross-section A and distance of unit length.
![]()
Molar conductivity increases with a decrease in concentration. This is because the total volume V of the solution containing one mole of the electrolyte increases on dilution.
Variation of molar conductivities with dilution:
For strong electrolytes, molar conductivity slowly increases with dilution.
For weak electrolytes, molar conductivity increases steeply on dilution, especially near lower concentrations. The variation of with for strong and weak electrolytes is shown in the following plot:

b) Given,
Conductivity, k = 0.146 x 10-3 S cm-1
Resistance, R = 1500 ohm

Express the relation between conductivity and molar conductivity of a solution held in a cell.
The molar conductivity of a solution at a given concentration is the conductance of volume V of a solution containing 1 mole of the electrolyte kept between two electrodes with the area of cross-section A and distance of unit length, the conductivity of the solution is given by the following relation.

The chemistry of corrosion of iron is essentially an electrochemical phenomenon. Explain the reactions occurring during the corrosion of iron in the atmosphere.
The process of corrosion is a redox reaction and involves simultaneous oxidation and reduction reactions. It can, therefore, be referred to as an electrochemical reaction.
In the process of corrosion, due to the presence of air and moisture, oxidation takes place at a particular spot of an object made of iron. That spot behaves as the anode. The reaction at the anode is can be written as follows.
Anode reaction: ![]()
Electrons released at the anodic spot move through the metallic object and go to another spot of the object. There, in the presence of H+ ions, the electrons reduce molecular oxygen. This spot behaves as the cathode. These H+ ions come either from H2CO3, which are formed due to the dissolution of carbon dioxide from the air into water or from the dissolution of other acidic oxides from the atmosphere in water.
The reaction corresponding at the cathode is written as follows.
Cathode reaction:
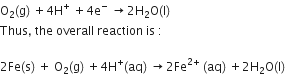
Also, ferrous ions are further oxidised by atmospheric oxygen to ferric ions.
These ferric ions combine with moisture, present in the surroundings, to form a hydrated ferric oxide (Fe2O3, x H2O) i.e., rust.
How much charge is required for the reduction of 1 mol of Zn2+ to Zn?
Zn2+ + 2e- ---> Zn
Number of electrons involved = 2
Charge required for the reduction of Zn2+ = 2F
We know
1F = 96,487 C
Thus,
2F = 2 x 96487 = 1,92,974 C
Define rate of reaction? Write two factors that affect the rate of reaction.
Rate of Reaction: rate of reaction may be defined as the change in concentration of a substance divided by the time interval during which this change is observed:
![]()
Factors Affecting Rate of Reaction
1. Concentration of reactants
2. Temperature
3. Catalyst
The conductivity of 0.20 mol L-1 solution of KCl is 2.48 x 10-2 S cm-1. Calculate its molar conductivity and degree of dissociation (K+) = 73.56 S cm2 mol-1 and (Cl-)= 76.5 S
(b) What type of battery is mercury cell? Why is it more advantageous than dry cell?
Conductivity of KCl solution = 2.48 x 10-2 S cm-1
Concentration of KCl solution = 0.20 mol L-1
= 0.20 x 1000 mol cm -3
= 200 mol cm-3
Molar conductivity

b) Mercury cell is a type of primary battery. In primary batteries , the charging reaction occurs only once and after it has been used over a period of time , the battery becomes dead and cannot be refused mercury cell is more advantage than dry cell has a very short life span due to the conversion of zinc to zinc chloride that makes the zinc casing porous . Due to this porous casing, the substance inside the cell leaks out and corrodes the metal, reducing the lifetime of the cell. While, in the case of mercury cell, the overall reaction does not involve the formation of any ion in the solution whose concentration can change during its life time.
The standard electrode potential (E°) for Daniell cell is +1·1 V. Calculate the G° for the reaction
Zn(s) + Cu2+ (aq) ----> Zn+ (aq) + Cu (s)
(1 F = 96500 C mol-1).
E° for Daniel cell = 1.1 V
Zn(s) + Cu2+ (aq)---> Zn2+ (aq) + Cu(s)
1F = 96500 C mol-1
G° =?
n = 2 (no. of e-s exchanged)
Since, G° = -nFE°
Therefore, G° = -2 x 96500 x 1.1
G° = -212300 J mol-1
G° = -212.3 kJ mol-1
Express the relation among cell constant, the resistance of the solution in the cell and conductivity of the solution. How is molar conductivity of a solution related to its conductivity?
The conductivity (k) of the solution in a cell is the reciprocal of its resistivity.
![]()
The quantity 1/2 is cell constant.
L --> Distance between 2 electrodes
a --> Area of cross section
R --> Resistance
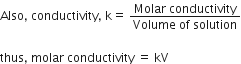
The electrical resistance of a column of 0.05 M NaOH solution of diameter 1cm and length 50
cm is 5.55 x 103 ohm. Calculate its resistivity, conductivity and molar conductivity.
A = πr2 = 3.14 x (0.5)2 cm2 = 0.785 cm2
l = 50 cm
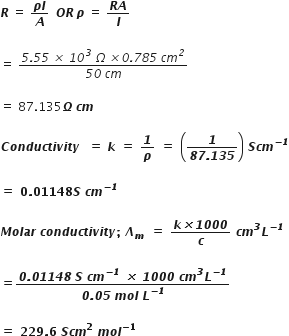
From the given cells:
Lead storage cell, Mercury cell, Fuel cell and Dry cell Ans the following:
(i) Which cell is used in hearing aids?
(ii) Which cell was used in Apollo Space Programme?
(iii)Which cell is used in automobiles and inverters?
(iv)Which cell does not have long life?
(i) Mercury cell is used in hearing aids.
(ii)Fuel cell was used in the Apollo space programme.
(iii)Lead storage cell is used in automobiles and inverters.
(iv) Dry cell does not have a long life.
a)
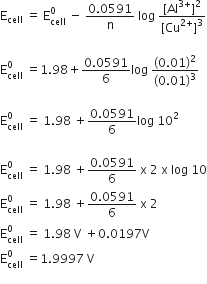
b) A is better for coating the surface of the iron because its E0 value is more negative.
Or
a) 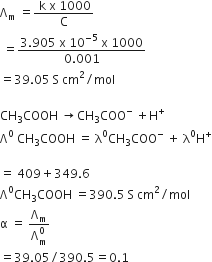
b) A device used for the production of electricity from energy released during the spontaneous chemical reaction and the use of electrical energy to bring about a chemical change. The reaction gets reversed / It starts acting as an electrolytic cell & vice – verse.
(a) What type of a battery is lead storage battery? Write the anode and cathode reactions and the overall cell reaction occurring in the operation of a lead storage battery.
(b) Calculate the potential for half-cell containing 0.10 M K2Cr2O7 (aq), 0.20 M Cr3+(aq) and 1.0 x 10-4 M H+ (aq)
The half-cell reaction is ![]()
And the standard electrode potential is given as E0 = 1.33 V.
OR
(a) How many moles of mercury will be produced by electrolysing 1.0 M?
Hg (NO3)2 solution with a current of 2.00 A for 3 hours?
[Hg (NO3)2 = 200.6 g mol-1]
(b) A voltaic cell is set up at 25°C with the following half-cells Al3+ (0.001 M) and Ni2+ (0.50 M). Write an equation for the reaction that occurs when the cell generates an electric current and determine the cell potential.
![]()
(a) A lead storage battery is a secondary battery.
The following chemical equations take place in a lead storage battery.
: 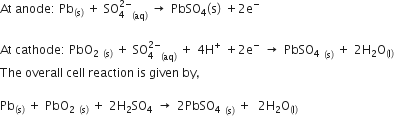
When a battery is charged, the reverse of all these reactions takes place.
Hence, on charging, PbSO4(s) present at the anode and cathode is converted into Pb(s) and PbO2(s) respectively.
b) 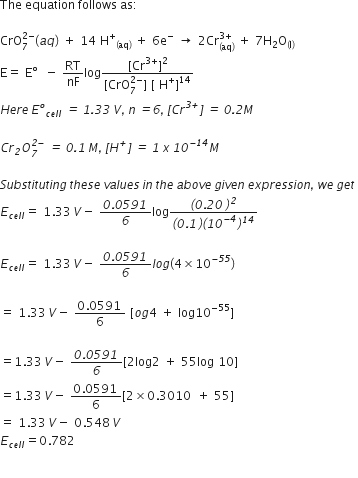
Or
(a) Quantity of electricity passed = (2A) x (3 x 60 x 60s) = 21600 C
Thus, 2F i.e. 2 x 96500 C deposit Hg = 1 mole 21600 C will deposit Hg
![]()
= 0.11 mole
Or
At anode: Al (s) --> Al3+ (aq) + 3e-] x2
At cathode: Ni2+ + 2e- --> Ni(s) ] x3
Cell reaction: 2Al(s) + 3Ni2+(aq) ---> 2Al3+(aq) + 3Ni (s)
Applying nernst equation to the above cell reaction
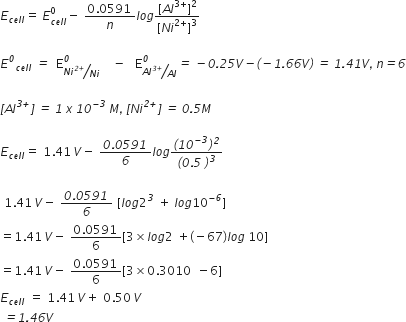
State Kohlrausch's law of independent migration of ions. Why does the conductivity of a solution decrease with dilution?
Kohlrausch's law of independent migration of ions: It states that the limiting molar conductivity of an electrolyte can be represented as the sum of the individual contributions of its anion and cation.
![]()
A conductivity of a solution decreases with dilution because it leads to decrease in a number of ions per unit volume.
(a) Calculate ![]() for the reaction
for the reaction
Mg (s) + Cu2+ (aq) → Mg2+ (aq) + Cu (s)
Given : E°cell = + 2.71 V, 1 F = 96500 C mol−1
(b) Name the type of cell which was used in Apollo space programme for providing electrical power.
(a) For the cell reaction,
Mg (s) + Cu2+ (aq) → Mg2+ (aq) + Cu (s) E°cell = + 2·71 V
The change in the standard Gibbs free energy is given as:
![]() = −nFE°cel. = −2 x 96500 x 2.71 = -523030 Jmol−1
= −nFE°cel. = −2 x 96500 x 2.71 = -523030 Jmol−1
b) The fuel cell which uses the reaction of hydrogen with oxygen to form water was used in Apollo space programme for providing electrical power.
Calculate the degree of dissociation (a) of acetic acid if its molar conductivity (Λm) is 39.05 S cm2mol–1. Given λo(H+) = 349.6 S cm2 mol–1 and λo(CH3COO–) = 40.9 S cm2 mol–1
The degree of dissociation is given by,
α = Λm / λo
here, α is the degree of dissociation,
Λm is the molar conductivity
λo is the molar conductivity at infinite dilution.
We have given
λo(H+) = 349.6 S cm2mol-1
and
λ0(CH3COO-) = 40.9 S cm2mol-1
then,
λoCH3COOH = λo CH3COO-+ λoH+
λoCH3COOH = 349.6 + 40.9 = 390.5
Now, degree of dissociation (α),
α = Λm /Λo
= 39.05 / 390.5 = 0.1
Calculate the mass of Ag deposited at cathode when a current of 2 amperes was passed through a solution of AgNO3 for 15 minutes.
(Given : Molar mass of Ag = 108 g mol–1 1F = 96500 C mol–1)
Given,
Molar mass of Ag = 108 g mol–1
1F = 96500 C mol–1
Q=It
Q= 2×15×60=1800 C
W(weight of substance deposited)= ZQ
Z= M/nF
n-factor here is 1
So Z = 108/1×96500
![]() =2.01 g
=2.01 g
The mass of Ag deposited at the cathode is2.01 g
Define fuel cell.
A fuel cell is a device that produces electrical energy through a chemical reaction between a source fuel and an oxidant.
Define fuel cell and write its two advantages.
These are voltaic cells in which the reactants are continuously supplied to the electrodes. There are designed to convert the energy from the combustion of a fuel like a hydrogen, methane, methanol, etc directly into electrical energy.
Advantage : -
(1) A fuel cell works with an efficiency of 60 to 70 %
(2) They are pollution free
Galvanization is applying a coating of:
-
Cr
-
Cu
-
Zn
-
Pb
C.
Zn
Zinc metal is the most stable metal to cover iron surfaces. The process of coating the iron surface by zinc is called galvanization.
Two Faraday of electricity is passed through a solution of CuSO4. The mass of copper deposited at the cathode is: (at. mass of Cu = 63.5 amu)
-
0 g
-
63.5 g
-
2 g
-
127 g
B.
63.5 g
Atomic mass of Cu = 63.5 u
Valency of the metal Z= 2
We have,
CuSO4 → Cu2+ + SO42-
Cu2+ + 2e- → Cu
Given below are the half-cell reactions
Mn2+ + 2e- → Mn; Eo = - 1.18 eV
2(Mn3+ + e- →Mn2+); Eo = +1.51 eV
The Eo for 3Mn2+ → Mn + 2Mn3+ will be
-
-2.69 V; the reaction will not occur
-
-2.69 V; the reaction will occure
-
-0.33 V; the reaction will not occur
-
-0.33 V; the reaction will occur
A.
-2.69 V; the reaction will not occur
Standard element potential of reaction [ Eo] can be calculated as
Eocell = ER-EP
where ER = SRP of reactant
EP = SRP of product
If Eocell = +ve, then the reaction is spontaneous otherwise non-spontaneous.
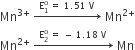
therefore, For Mn2+ disproportionation
Eo = - 1.51 V - 1.18 V = - 2.69 V < 0
Given,
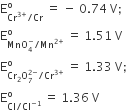
Based on the data given above, strongest oxidising agent will be
-
Cl
-
Cr3+
-
Mn2+
-
MnO4-
D.
MnO4-
Higher the SRP, better is an oxidising agent, among the given ![]() is highest, hence, MnO4- is a strongest oxidising agent.
is highest, hence, MnO4- is a strongest oxidising agent.
How many litres of water must be added to 1 L to an aqueous solution of HCl with a pH of 1 create an aqueous solution with PH of 2?
-
0.1 L
-
0.9 L
-
2.0 L
-
9.0 L
D.
9.0 L
Initial pH = 1, i.e. [H+] = 0.1 mole/litre
New pH = 2, i.e. [H+] = 0.01 mole/litre
In case of dilution: M1V1 = M2V2
0.1 ×1 =0.01 × V2
V2 = 10 litre.
A volume of water added = 9 litres.
The reduction potential of hydrogen half-cell will be negative if
-
p(H2) = 1 atm and [H+] = 2.0 M
-
p(H2) = 1 atm and [H+] = 1.0 M
-
p(H2) = 2 atm and [H+] = 1.0 M
-
p(H2) = 2 atm and [H+] = 2.0 M
C.
p(H2) = 2 atm and [H+] = 1.0 M
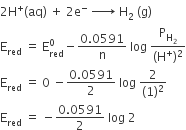
Thus correct answer is c.
Given,
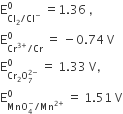
Among the following, the strongest reducing agent is
-
Cr
-
Mn2+
-
Cr3+
-
Cl-
A.
Cr
Cr+3 is having least reducing potential, therefore Cr is the best Reducing agent.
On the basis of the following thermochemical data: (∆f G° H(aq)+=0)
H2O(l) → H+(aq) + OH–(aq); ∆H =57.32kJ
H2(g) + 1/2O2(g) → H2O(l); ∆H = –286.20 kJ
The value of enthalpy of formation of OH–ion at 25°C is
-
–22.88 kJ
-
–228.88 kJ
-
+228.88 kJ
-
–343.52 kJ
B.
–228.88 kJ
By adding the two given equations, we have
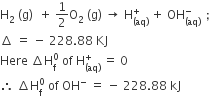
Aluminium oxide may be electrolysed at 1000o C to furnish aluminium metal (Atomic mass = 27 amu; 1 Faraday = 96,500 Coulombs). The cathode reaction is
Al3+ +3e- → Alo
To prepare 5.12 kg of aluminium metal by this method would require
-
5.49 x 107 C of electricity
-
1.83 x 107 C of electricity
-
1.49x 107 C of electricity
-
5.49 x 101 C of electricity
A.
5.49 x 107 C of electricity
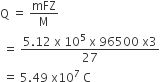
Calomel (Hg2Cl2) on reaction with ammonium hydroxide gives
-
HgNH2Cl
-
NH2 – Hg – Hg – Cl
-
Hg2O
-
HgO
A.
HgNH2Cl
Hg2Cl2 + 2NH4OH → Hg + Hg(NH2)Cl + NH4Cl + 2H2O
Which among the following factors is the most important in making fluorine the strongest oxidizing halogen?
-
Electron affinity
-
Bond dissociation energy
-
Hydration enthalpy
-
Ionization enthalpy
B.
Bond dissociation energy
Oxidising power depends on the (+ve) reduction potential value, and (-ve) value of Gibbs free energy.
F2 has the most negative ΔG° value which dependent on hydration enthalpy.
In hydrogen-oxygen fuel cell, combustion of hydrogen occurs to
-
generate heat
-
remove adsorbed oxygen from electrode surfaces
-
produce high purity water
-
create potential difference between the two electrodes
D.
create potential difference between the two electrodes
Any cell (such as fuel cell), works when a potential difference is developed.
The standard e.m.f of a cell, involving one electron change is found to be 0.591 V at 25°C. The equilibrium constant of the reaction is (F = 96,500 C mol-1: R = 8.314 JK-1 mol-1)
-
1.0×101
-
1.0×1030
-
1.0×1010
-
1.0×1010
D.
1.0×1010
Relation between Keq and Ecell is
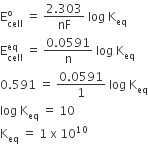
The limiting molar conductivities Λ° for NaCl, KBr and KCl are 126, 152 and 150 S cm2 mol-1 respectively. The Λ° for NaBr is
-
128 S cm2 mol-1
-
302 S cm2 mol-1
-
278 S cm2 mol-1
-
176 S cm2 mol-1
A.
128 S cm2 mol-1
By Kohlrausch's law
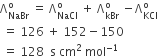
In a cell that utilises the reaction Zn(s) + 2H+ (aq) → Zn2+(aq) + H2(g) addition of H2SO4 to cathode compartment, will
-
lower the E and shift equilibrium to the left
-
increases the E and shift equilibrium to the left
-
increase the E and shift equilibrium to the right
-
Lower the E and shift equilibrium to the right
C.
increase the E and shift equilibrium to the right
The ![]() values for Cr, Mn, Fe and Co are – 0.41, +1.57, + 0.77 and +1.97 V respectively. For which one of these metals the change in oxidation state form +2 to +3 is easiest?
values for Cr, Mn, Fe and Co are – 0.41, +1.57, + 0.77 and +1.97 V respectively. For which one of these metals the change in oxidation state form +2 to +3 is easiest?
-
Cr
-
Co
-
Fe
-
Mn
A.
Cr
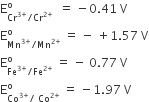
More negative value of
How long (approximate) should water be electrolysed by passing through 100 amperes current so that the oxygen released can completely burn 27.66 g of diborane? (Atomic weight of B = 10.8 u)
1.6 hours
6.4 hours
0.8 hours
3.2 hours
D.
3.2 hours
According to the balanced equation:
27.66 g B2H6 i.e. 1 mole B2H6 requires 3 moles of O2. Now, this oxygen is produced by electrolysis of water.
1 mole O2 is produced by 4 F charge
therefore, 3 mole O2 will be produced by 12 F charge
hence, Now applying
Q = It
12 x 96500 C = 100 x t (s)
t = 3.2
The pressure of H2 required to make the potential of H2-electrode zero in pure water at 298 K is
-
10-12 atm
-
10-10 atm
-
10-4 atm
-
10-14 atm
D.
10-14 atm
From the question, we have an equation
2H+ +2e- --> H2(g)
According to Nernst equation

A device that converts energy of combustion of fuels like hydrogen and methane, directly into electrical energy is known as
-
fuel cell
-
electrolytic cell
-
dynamo
-
Ni-Cd cell
A.
fuel cell
A fuel cell is a device that converts the energy of combustion of fuels like hydrogen and methane, directly into electrical energy.
Electrolytic cell converts electrical energy into chemical energy. Dynamo is an electrical generator that produces direct current with the use of a commutator.
Ni-Cd cell is the type of rechargeable battery which consists of a cadmium anode and a metal grid containing NiO2 acting as a cathode.
When 0.1 mol MnO42- is oxidised, the quantity of electricity required to completely oxidise MnO42- to MnO42- is
-
95600C
-
2 x 96500C
-
9650 C
-
96.50 C
C.
9650 C
As per the equation, for 1 mole of MnO42-, 1F of electricity is required. Thus, for 0.1 mole of MnO4-, 0.1 F of electricity is required.
Since, 1 F = 96500 C
0.1 F = 0.1 x 96500 C
= 9650 C
Hence, 9650 C of electricity is required to completely oxidise MnO42- to MnO4-
Using the Gibbs energy change, ΔG0 = +63.3 KJ for the following reaction,
Ag2CO3 (s) r2Ag+ (aq) + CO32- (aq)
the Ksp of Ag2CO3 (s) in water at 250 C is (R= 8.314 JK-1 mol-1)
-
3.2 x 10-26
-
8.0 x 10-12
-
2.9 x 10-3
-
7.9 x 10-2
B.
8.0 x 10-12
ΔG0 is related to Ksp by the equation,ΔG0 = --2.303 RT log Ksp
We have Given,
ΔG0 = +63.3 KJ = 63.3 x 103 J
Thus, substitute ΔG0 = 63.3 x 103 J
R = 8.314 JK-1 mol-1
and T =298 K [ 25 +273 K] into the above equation to get,
63.3 x 103 = -2.303 x 8.314 x 298 log Ksp
log Ksp = -11.09
Ksp = antilog (-11.09)
Ksp = 8.0 x 10-12
At 250 molar conductance of 0.1 molar aqueous solutions of ammonium hydroxide is 9.54 ohm-1 cm2 mol-1 and at infinite dilution, its molar conductance is 238 ohm-1 cm2 mol-1. The degree of ionisation of ammonium hydroxide at the same concentration and temperature is
-
2.080%
-
20.800%
-
4.008%
-
40.800%
C.
4.008%
Given molar conductance at 0.1 M concentration,
λc = 9.54 Ω-1 cm2 mol-1
Molar conductance at infinite dilution,
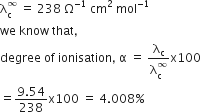
A button cell used in watches functions as following
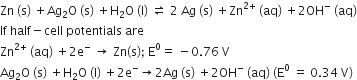
The cell potential will be
-
1.10 V
-
0.42 V
-
0.84 V
-
1.34 V
A.
1.10 V
Anode is always the site of oxidation thus anode half cell is
Zn2+ (aq) +2e- --> Zn (s); E0 =-0.76 V
Cathode half cell is
Ag2O (s) +H2O (l) +2e- ---> 2Ag(s) +2OH- (aq); E0 =0.34 V
E0cell = E0cathode -E0anode
= 0.34 -(-0.76) = +1.10 V
Limiting molar conductivity of NH4OH (i.e., ![]() is equal to
is equal to
D.
According to Kohlrausch's law, limiting molar conductivity of NH4OH
![]()
Standard reduction potentials of the half-reactions are given below.
F2 (g) +2e- → 2F- (aq) ; Eo = +2.85 V
Cl2 (g) +2e- →2Cl- (aq) ; Eo = +1.36V
Br2 (l) +2e- → 2Br- (aq) ; Eo = +1.06 V
I2 (s) +2e- →2I- (aq); Eo = +0.53 V
The strongest oxidising and reducing agents respectively are
-
F2 and I-
-
Br2 and Cl-
-
Cl2 and Br-
-
Cl2 and I2
A.
F2 and I-
Higher the value of standard reduction potential, stronger will be the oxidising agent. Therefore, F2 will act as stronger oxidising agent.
Similarly, lower the value of standard reduction potential stronger will be the reducing agent. Therefore, I- will act as strongest reducing agent.
Molar conductivities (Λom) at infinite dilution of NaCl, HCl and CH3COONa are 126.4, 425.9 and 91.0 S Cm2 mol-1 respectively. Λom for CH3COOH will be
-
425.5 S cm2 mol-1
-
180.5 S cm2 mol-1
-
290.8 S cm2 mol-1
-
390.5 S cm2 mol-1
D.
390.5 S cm2 mol-1
CH3COONa +HCl → NaCl + CH3COOH
91 + 425.9 = 126.4 + x
x= 516.9 -126.4
= 390.5 S cm2 mol-1
The Gibb's energy for the decomposition of Al2O3 at 500o C is as follow
2/3 Al2O3 → 4/3 Al + O2;
ΔrG = +960 kJ mol-1
The potential difference needed for the electrolytic reduction aluminium oxide (Al2O3) at 5000 C is at least
-
4.5 V
-
3.0 V
-
2.5 V
-
5.0 V
C.
2.5 V
at anode
2O2- +4e- →O2] x 3
at cathode
Al3+ → Al +3e-] x4
Net reaction
4Al +6O2- → 3O2 +4Al
Or
4/3Al +2O2- → O2 +4/3Al
n= 12/3 = 4
ΔGo = -nFEo
ΔGo = +960 kJ mol-1
= 960 x 1000 J mol-1
n=4
F =96500 Coulomb
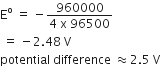
Standard electrode potential of three metal X, Y and Z are -1.2 V, +0.5 V and -3.0 V respectively. The reducing power of these metals will be
-
Y > X > Z
-
Z> X> Y
-
X > Y > Z
-
Y > Z > X
B.
Z> X> Y
Exo = -1.2 V;
therefore, Z > X > Y
Because, higher the reduction potential, lesser the reducing power.
If the Eocell for a given reaction has a negative value then which of the following gives the correct relationships for the values of ΔGo and Keq ?
-
ΔGo < 0; Keq > 1
-
ΔGo < 0; Keq < 1
-
ΔGo > 0; Keq < 1
-
ΔGo > 0; Keq > 1
C.
ΔGo > 0; Keq < 1
ΔGo = - nFEo
When Eo is negative, then ΔGo > 0
ΔGo = - RT ln Keq
When ΔGo >0, Keq = 10-x which is less than ones ,ie, Keq < 1.
A solution contains Fe2+ Fe3+, and I- ions. This solution was treated with iodine at 35o C.Eo for Fe3+/ Fe2+ is +0.77 V and Eo for I2/ 2 I- = 0.536 V. The favourable redox reaction is
-
I2 will be reduced to I-
-
There will be No redox reaction
-
I- will be oxidised to I2
-
Fe2+ will be oxidised to Fe3+
C.
I- will be oxidised to I2
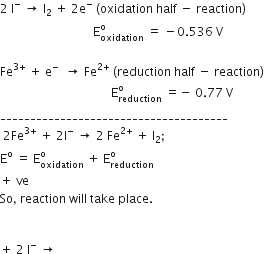
For the reaction of silver ions with copper metal, the standard cell potential was found to be +0.46 V at 25o C. The value of standard Gibbs energy, ΔGo will be (F = 96500 C mol-1 )
-
-89.0 kJ
-
-89.0 J
-
-44.5 kJ
-
-98.0 kJ
A.
-89.0 kJ
We know that,
standard Gibbs energy, ΔGo = -nFEocell
For the cell reaction,
ΔEocell =+ 0.46 V
ΔGo = =- 2 x 96500 x 0.46
= -88780 J
=-88.7 kJ = -89.0 kJ
An increase in equivalent conductance of strong electrolyte with dilution is mainly due to
-
the increase in ionic mobility of ions
-
100% ionisation of electrolyte at normal dilution
-
the increase in both, ie, the number of ions and ionic mobility of ions
-
the increase in the number of ions
A.
the increase in ionic mobility of ions
on dilution, the number of current carrying particles per cm3 decreases but the volume of solution increases. Consequently, the ionic solution increases. Consequently, the ionic mobility increases, which in turn increases the equivalent conductance of strong electrolyte.
Which of the following expression correctly represents the equivalent conductance at infinite dilution of Al2(SO4)3. Given that ![]() are the equivalent conductance at infinite dilution of the respective ions.
are the equivalent conductance at infinite dilution of the respective ions.
B.
Al2(SO4)3 ⇌ 2 Al3+ + 3SO42-
Since equivalent conductances are given only for ions, the equivalent conductance at infinite dilution,
![]()
For vaporisation of water at 1 atm pressure, the values of ΔH and ΔS are 40.63 kJ mol- and 108.8 JK-1 mol-1, respectively. The temperature when Gibbs energy change (ΔG) for this transformation will be zero, is
-
273.14 K
-
393.4 K
-
373.4 K
-
293.4 K
C.
373.4 K
ΔG = ΔH - TΔS
ΔG = 0 (given)
ΔH = TΔS,
T = 40.63 x 103 / 108.8 = 373.4 K
Al2O3 is reduced by electrolysis at low potentials and high currents. If 4.5 x 104 An of current is passed through molten Al2O3 for 6h, what mass of aluminium is produced? (Assume 100% current efficiency, at.mass of Al = 27 g mol-1)
-
9.0 x 103 g
-
8.1 x 104 g
-
2.4 x 105 g
-
1.3 x 104 g
B.
8.1 x 104 g
Al2O3 ionises as,
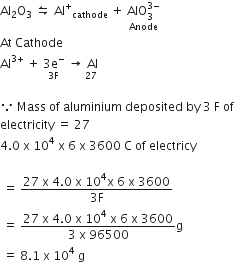
The equivalent conductance of M/32 solution of weak monobasic acid is 8.0 ohm-cm2 and at infinite dilution is 400 ohm-cm2. The dissociation constant of this acid is
-
1.25 x 10-5
-
1.25 x 10-6
-
6.25 x 10-4
-
1.25 x 10-4
A.
1.25 x 10-5
Degree of dissociation,
![]()
Where, ![]() and
and ![]() are equivalent conductances at a given concentration and at infinite dilution respectively.
are equivalent conductances at a given concentration and at infinite dilution respectively.
![]()
From Ostwald's dilution law (for weak monobasic acid)
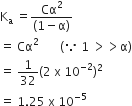
Given,
(i) Cu2+ + 2e- → Cu, Eo = 0.337 V
(ii) Cu2+ +e- → Cu+, Eo = 0.153
Electrode potential, Eo for the reaction,
Cu +e- →Cu, will be
-
0.52 V
-
0.90 V
-
0.30 V
-
0.38 V
A.
0.52 V
Gibb's free energy is an additive property.
ΔGo = -nFEo
For reaction, Cu2+ +2e- → Cu;
ΔGo = 2 x F x 0.337 ... (i)
For reaction, Cu+ → Cu2+ +e-;
ΔGo = +1 xF x 0.153
Adding Eqs. (i) and (ii), we get
Cu+ + e- → Cu; ΔGo = - -0.521 F
ΔGo = - nFEo
-0.521 F = -nFEo
Eo = 0.52 V
The values of ΔH and ΔSfor the reaction, C(graphite) + CO2 → 2 CO (g) are 170 kJ and 170 JK-1 respectively. This reaction will be spontaneous at
-
710 K
-
910 K
-
1110 K
-
510 K
C.
1110 K
For spontaneous process, ΔG < 0
ΔG = ΔH - TΔS
Given , ΔH = 170 KJ = 170 x 103 J
ΔS = 170 JK-1
T = ?
ΔG = ΔH - TΔS
0 < 70 x 103 - T x 170
T > 1000
T = 1100 K
Kohlrausch's law states that at
-
finite dilution, each ion makes a definite contribution to the equivalent conductance of an electrolyte, whatever be the nature of the other ion of the electrolyte.
-
infinite dilution, each ion makes a definite contribution to the equivalent conductance of an electrolyte depending on the nature of the other ion of the electrolyte.
-
infinite dilution, each ion makes a definite contribution to the conductance of an electrolyte whatever be the nature of the other ions of the electrolyte.
-
infinite dilution, each ion makes a definite contribution to the equivalent conductance of an electrolyte. whatever be the nature of the other of the electrolyte.
D.
infinite dilution, each ion makes a definite contribution to the equivalent conductance of an electrolyte. whatever be the nature of the other of the electrolyte.
According to Kohlrausch's law 'at infinite dilution when the dissociation is complete, each ion makes a definite contribution towards equivalent condctivity of the electrolyte irrespective of the nature of the order ion with which it is associated.
The equilibrium constant of the reaction:
Cu (s) + 2 Ag+ (aq) → Cu2+ (aq) + 2 Ag (s);
Eo = 0.46 V at 298 K
-
2.4 x 1010
-
2.0 x 1010
-
4.0 x 1010
-
4.0 x 1015
D.
4.0 x 1015
Cu (s) + 2 Ag+ (aq) → Cu2+ (aq) + 2 Ag (s)
Eo = 0.46 V at 298 K
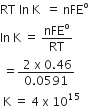
If ![]() the standard emf of the reaction:
the standard emf of the reaction:
Fe + 2 Fe3+ →3Fe2+
will be:
-
0.330 V
-
1.653 V
-
1.212 V
-
0.111 V
C.
Given that,
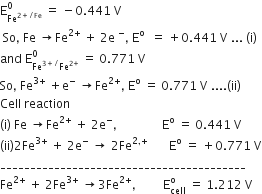
So, on the basis of cell reaction following half-cell reactions are written
At anode:
Fe → Fe2+ + 2e- (oxidation)
At cathode:
2Fe3+ + 2e- →2Fe2+ (reduction)
So,
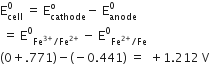
A hypothetical electrochemical cell is shown below
A- | A+ (xM)|| B+ (yM)|B+
The emf measured is +0.20 V. The cell reaction is:
-
A+ + B → A + B+
-
A+ + e- → A ; B+ + e- → B-
-
the cell reaction cannot be predicted
-
A+ + B → A+ + B
D.
A+ + B → A+ + B
Electrochemical cell
A0 | A+ (xM) ||B+ yM) B+
The emf of cell is +.20 V. so cell reaction is possible. The half cell reaction are given as follows:
(i) At negative pole:
A → A+ + e- (oxidation)
(ii) At positive pole:
B+ + e- → B (reduction)
A +B- → A+ + B-, Ecello = + 0.20 V
In the electrochemical cell :
Zn|ZnSO4(0.01M)||CuSO4(1.0 M)|Cu, the emf of this Daniel cell is E1. When the concentration of ZnSO4 is changed to 1.0 M and that of CuSO4 changed to 0.01 M, the emf changes to E2. From the following, which one is the relationship between E1 and E2?
(Given, RT/F= 0.059)
-
E1= E2
-
E1< E2
-
E1> E2
-
E2= 0 ≠ E1
C.
E1> E2
Zn|ZnSO4(0.01 M)||CuSO4(1.0 M)|Cu
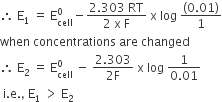
When a lead storage battery is discharged;
SO2 is evolved
lead sulphate is consumed
lead is formed
sulphuric acid is consumed
D.
sulphuric acid is consumed
H2SO3 is consumed during discharging of the lead storage battery as Pb changes PbSO4 and PbO2 changes to PbSO4.
The value of reaction quotient [Q], for the following cell
Zn(s)|Zn2+ (0.01 M)|| Ag+ (1.25 M | Ag (s) is
156
125
1.25 x 10-2
6.4 x 10-3
D.
6.4 x 10-3
The cell reaction is
Zn(s) → Zn2+ (0.01 M) + 2e-
[Ag+ (1.25 M) + e- → Ag (s)] x 2
________________________________________
Zn(s) + 2Ag+ (1.25 M) → Zn2+ (0.01 M) + 2Ag(s)
value of Q = 6.4 x 10-3
The standard reduction potential for Zn2+/Zn, Ni2+/Ni and Fe2+/Fe are -0.76, -0.23 and -0.44 V, respectively.
The reaction X + Y2+ → X2+ + Y will be spontaneous when
X = Ni, Y = Fe
X = Ni, Y = Zn
X =Fe, Y = Zn
X = Zn, Y = Ni
D.
X = Zn, Y = Ni
The reaction is spontaneous when ΔG < 0 and E°cell > 0.
Mock Test Series
Sponsor Area
Sponsor Area