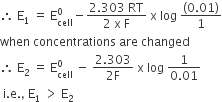Question
In the electrochemical cell :
Zn|ZnSO4(0.01M)||CuSO4(1.0 M)|Cu, the emf of this Daniel cell is E1. When the concentration of ZnSO4 is changed to 1.0 M and that of CuSO4 changed to 0.01 M, the emf changes to E2. From the following, which one is the relationship between E1 and E2?
(Given, RT/F= 0.059)
-
E1= E2
-
E1< E2
-
E1> E2
-
E2= 0 ≠ E1
Solution
C.
E1> E2
Zn|ZnSO4(0.01 M)||CuSO4(1.0 M)|Cu