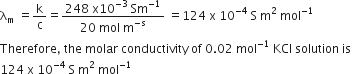The resistance of a conductivity cell filled with 0.1 mol L-1 KCl solution is 100 . If the resistance of the same cell when filled with 0.02 mol L-1 KCl solution is 520 ![]() , calculate the conductivity and molar conductivity of 0.02 mol L-1 KCl solution. The conductivity of 0.1 mol L-1 KCl solution is 1.29x 10-2
, calculate the conductivity and molar conductivity of 0.02 mol L-1 KCl solution. The conductivity of 0.1 mol L-1 KCl solution is 1.29x 10-2 ![]() -1cm-1
-1cm-1
Given that:
Concentration of the KCl solution = 0.1 mol L-1
Resistance of cell filled with 0.1 mol L-1 KCl solution = 100 ohm
Cell constant = G* = conductivity x resistance
1.29x10-2 ohm-1 cm-1 x 100 ohm = 1.29 cm-1 = 129 m-1
Cell constant for a particular conductivity cell is a constant.
Conductivity of 0.02 mol L-1 KCl solution = =0.248 Sm-1
Concentration = 0.02 mol-1
= 1000x 0.02 mol m-3 = 20 mol m-3
Now,
Molar conductivity =