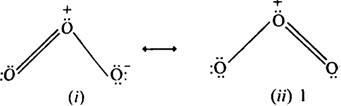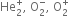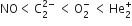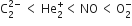Chemistry Part I Chapter 4 Chemical Bonding And Molecular Structure
Sponsor Area
NCERT Solution For Class 11 Political%25252bscience Chemistry Part I
What are valence electrons?
Why do atoms combine?
Atoms combine because they tend to complete their octets or duplets in the case of H, Li and Be. Another reason for the combination of atoms is the lowering of enthalpy that takes place when atoms combine together.
Why two helium atoms do not combine to form He2?
(i) Each helium atom has a fully filled orbital whereas only half filled atomic orbitals combine to form a bond.
(ii) When two helium atoms approach each other, new forces of repulsion are greater than the forces of attraction.
What are 'Lewis structures'?

Name the conditions for the formation of an ionic bond between two atoms.
The conditions for the formation of an ionic bond:
(i) One of the atoms should have low ionisation enthalpy.
(ii) The second atom should have negative electron gain enthalpy.
What is crystal lattice?
Crystal lattice is well defined three-dimensional network, in which each ion is surrounded by oppositely charged ions in a regular pattern.
What is electrovalency?
Write Lewis dot symbols for atoms of the elements Mg, Na, B, O, N, Br.
Lewis dot structure of given elements are:
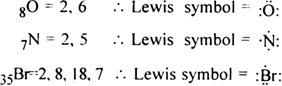
Draw the Lewis structure for the ionic compound by aluminium and fluorine.
Lewis structure of the aluminium fluoride.![]()
Two elements A and B have the electronic configuration as:
A = 1s22s22p63s2 and B = 1s22s22p5
Write the empirical formula of the substance containing A and B.
What type of bonding would you expect between:
(i) a metal and a non-metal
(ii) a non-metal and another non-metal?
(ii) covalent.
Give reasons in one or two sentences for the observation that in their compounds non-metals form anions and not cations.
Out of Na and K, which will form a more stable ionic bond?
Which will have a greater lattice enthalpy: NaCl or MgO?
Is a covalent molecule always formed between two similar atoms?
Sponsor Area
Why sodium chloride is a solid whereas carbon tetrachloride is a liquid?
Why NaCl gives a white percipitate with AgNO3 solution but CCl4 does not?
NaCl is an ionic compound which gives Na+ and Cl– ions in aqueous solution. Cl- ions combine with Ag+ ions of AgNO3 to form a precipitate of AgCl. On the other hand, CCl4 is a covalent compound and does not give Cl ions.
What is covalency of an element?
Identify the types of bonds that you would expect to find in the molecules of each of the following substances:
water, ammonia and sodium chloride.
The type of bonds that present in the given molecules is,
Covalent: water and ammonia ;
Ionic bond: sodium chloride.
Why covalent compounds show isomerism but ionic compounds do not?
Three elements are having the following Lewis symbols:![]()
Write the formulae and Lewis structure of the covalent compounds formed between:
(i) A and B and (ii) A and C.
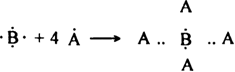

(ii) Covalent bond between A and C:
Give the types of bond in oxygen and nitrogen molecules.
The skeletal structure of CH3COOH as shown below is correct, but some of the bonds are shown incorrectly. Write the correct Lewis structure for acetic acid:


Arrange LiCl, BCl3, BeCl2 and CCl4 in order of increasing covalent bond character.
This is because Cl being fixed, electro-negativity of Li < Be < B < C so that electronegativity difference with chlorine decreases.
What important characteristics should M and X have so that they form a predominantly ionic compound MX?
Why is sigma bond stronger than Pi bond?
Because overlapping along the internuclear axis is greater than the side-wise overlapping.
How many sigma ![]() and
and ![]() bonds are present in a molecule of tetracyanoethylene?
bonds are present in a molecule of tetracyanoethylene?

Number of sigma bonds = 9;
A number of Pi bonds = 9.
What is the total number of sigma and pi bonds in the following molecules?
(a) C2H2 (b) C2H4
(a) C2H2
H – C ≡ C – H
Number of sigma bonds = 3
Number of ![]() bonds = 2
bonds = 2
Number of sigma bonds = 5
Number of ![]() bonds = 1
bonds = 1
What type of bonding is present in HCl?
Amongst LiCl, RbCl, BeCl2 and MgCl2, select the compound having:
(i) greatest ionic character
(ii) least ionic character.
(i) Greatest ionic character: RbCl
(ii) Least ionic character: BeCl2
How sigma bond formed?
Sigma bonds (σ bond) are the strongest type of covalent chemical bond. They are formed by head-on-head overlapping between atomic orbitals.
How is n bond formed?
What is meant by internuclear axis?
What is the use of VSEPR theory?
Mention the shape of NH3 and H2O.
The shape of the given compound:
NH3 = Trigonal Pyramidal.
H2O = Bent Shape.
Sponsor Area
Arrange the following molecules in order of increasing bond angles around the central atom: BeF2, BF3, CH4, NH3, H2O.
Predict the shapes of the given covalent molecules on the basis of VSEPR theory: AlCl3, PH3, CCl4.
(ii) PH3 : Pyramidal
(iii) CCl4 : Tetrahedral.
Give the order of repulsion among period orbitals?
Lone pair-lone pair > Lone pair-bond pair > Bond pair-bond pair.
Which of the two P - Cl and P - I bond is more polar
Arrange the following in the decreasing order of their polarity: HF, Hl, HCl, HBr.
HF> HCl > HBr > Hl.
What is the unit of dipole moment?
1 Debye = 10 – 18 e.s.u. cm.
How is the dipole moment of a polyatomic molecule determined?
The dipole moment of CO2 is zero. Why?
The dipole moment of NH3 is 1.49 D. Can you predict the shape?
Explain why BeH2 molecule has a zero dipolemoment although the Be-H bonds are polar.

Can a non-polar molecule have polar covalent bonds? Give two examples.
The molecule of SO2 has a dipole moment. Is the molecule linear or bent?
What is the dipole moment of BF3 or CH4 or CCl4? What do you conclude from this value?
BF3, CH4, and CCl4 molecules: In BF3, three are three BF bonds which are oriented at an angle of 1200 to one another. The three B-F bonds lie in one plane and cancel the dipole moment of one another. Hence, the dipole moment of BF3 would be zero. Similarly, the dipole moments of methane (CH4) and carbon tetrachloride (CCl4) molecules would be zero due to their symmetrical tetrahedral shape.
Arrange the bonds in order of increasing ionic character in the molecules:
LiF, K2O, N2, SO2 and ClF3.
Dipole moment helps in calculating the percentage ionic character of polar bonds. It is the ratio of observed dipole moment to the dipole moment for the complete transfer of electrons. Greater the difference in electronegativity of bonding atoms, the greater will be the ionic character.
On this basis, the order of increasing ionic character in the given molecules is,
N2 < SO2 < ClF3 < K2O < LiF
Write the electronic configuration of carbon atom in the excited state.
The electronic configuration of carbon atom in the excited state given as 1s22s22p1 .
What is the percentage of s character in sp3 hybridised orbital?
Account for the bond angle of 180° in BeCl2 molecule?
Out of ethane, ethene and ethyne, which has shorter carbon-carbon bond length?
What is Sigma bond ?
This type of covalent bond is formed by the end to end (hand-on) overlap of bonding orbitals along the internuclear axis. This is called as head on overlap or axial overlap.
How many ![]() bonds are present in the molecules of:
bonds are present in the molecules of:
(i) O2 and (ii) N2?
(i) O2: One sigma and one ![]() bond
bond
(ii) N2: One sigma and two ![]() bonds.
bonds.
What type of bond is possible between a filled orbital and an empty orbital?
Draw the shapes of the following hybrid orbitals: sp, sp2, sp3.
Shapes of the sp, sp2, sp3.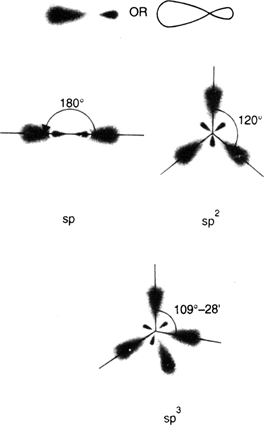
What is a hybrid orbtial?
Hybrid orbitals are types of atomic orbital that result when two or more atomic orbitals of an isolated atom mix such as the number of hybrid orbital on a covalently bonded atom are equal to the number of atomic orbitals used to form the hybrid orbitals.
Hybrid orbitals are usually involved in sigma bonds in polyatomic molecules; pi bonds usually involve the overlap of hybridised orbitals.
. For example, if one s –and p – orbitals intermix and redistribute their energies, two equivalent sp orbitals are formed called hybrid orbitals.Sponsor Area
Name the hybridization and the orbitals involved in the shape of CCl4 molecule.
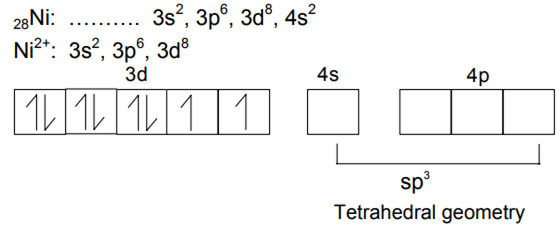
Name the hybridization and orbitals involved in the shape of |Ni(CN)4|2-.
What types of shapes are associated with sp3d and sp3d2 orbitals?
(i) sp3d – Trigonal bipyramidal.
(ii) sp3d2 – octahedral.
Which type of hybridization explains the trigonal bipyramidal shape of SF4 ?
What is the geometry of:
(i) PF5 molecule
(ii) SF6 molecule?
(i) PF5 molecule has trigonal bipyramidal geometry.
(ii) SF6 molecule has octahedral geometry.
Why all P-F bonds in PFS molecule are not of the same length?
What are the molecular shapes of:
(i) [Ni(CN)4]2– and
(ii) PCl5?
(i) [Ni(CN)4]2-: Square planar.
(ii) PCl5: Trigonal bipyramidal.
Name the hybridization and the orbitals involved in the shape of SF6 molecule ?
sp3d2 hybridization involved in the shape of SF6 molecule. The geometry of SF6 molecule is octahedral and the orbitals involved are 3s, 3py, 3py, 3pz and 3dx2-y2 and 3dz2.
What type of hybridization carbon atom has in (i) diamond (ii) graphite?
(i) Diamond sp3: hybridisation,
(ii) Graphite : sp2 hybridisation
What is molecular orbital?
What is a bonding molecular orbital?
Ψb = ΨA +Ψb
where ΨA and ΨB are the wave functions of the two combining atoms.
What is an antibonding molecular orbital?
The molecular orbital formed by the subtraction of electron density of two atomic orbitals is called antibonding molecular orbital. In terms of wave function, the antibonding MO is expressed as
Antibonding = ΨA – ΨB.
Name the molecular orbitals formed by the combination of two atomic orbitals.

How many molecular orbitals (M.O’s) of H2 can be formed from the hydrogen Is atomic orbitals?
Two molecular orbitals can be formed.
One is bonding molecular orbital (σ 1s) and the other is antibonding molecular orbital (σ*1s).
What type of atomic orbital can overlap to form molecular orbitals?
How is bonding molecular orbital in a molecule of hydrogen different from its antibonding molecular orbtial?
What is meant by bond order?
How do you express the bond length is terms of bond order?
In a molecule as we increase the number of electrons shared between two atoms, there will be an increase in bond order, also there will be an increase in the strength of the bond, and decrease the distance between nuclei. For example,
|
Bond |
No. of electron |
Bond order |
Bond strength |
Bond Length |
|
Single |
2 |
1 |
Weakest |
Longest |
|
Double |
4 |
2 |
- |
- |
|
Triple |
6 |
3 |
Strongest |
Shortest |
How is stability related to bond order?
Can we differentiate among single, double and triple bonds in terms of bond order?
|
Bond |
No. of electron |
Bond order |
Bond strength |
Bond Length |
|
Single |
2 |
1 |
Weakest |
Longest |
|
Double |
4 |
2 |
- |
- |
|
Triple |
6 |
3 |
Strongest |
Shortest |
How is bond order related to bond length and dissociation energy of a molecule?
Which has more bond dissociation energy and why: O+2 or O2?
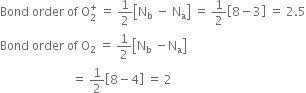
∵ Bond order of O+2 > Bond order of O2
∴ Bond dissociation energy of O+2 > Bond dissociation energy of O2.
Why is hydrogen molecule more stable than hydrogen atom?
How is bond length related to the stability of a molecule?
Which one of the two O+2 and O-2 has higher bond order and why?
![<pre>uncaught exception: <b>Http Error #404</b><br /><br />in file: /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/com/wiris/plugin/impl/HttpImpl.class.php line 61<br />#0 [internal function]: com_wiris_plugin_impl_HttpImpl_0(Object(com_wiris_plugin_impl_HttpImpl), NULL, 'http://www.wiri...', 'Http Error #404')
#1 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/php/Boot.class.php(769): call_user_func_array('com_wiris_plugi...', Array)
#2 [internal function]: _hx_lambda->execute('Http Error #404')
#3 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/haxe/Http.class.php(532): call_user_func_array(Array, Array)
#4 [internal function]: haxe_Http_5(true, Object(com_wiris_plugin_impl_HttpImpl), Object(com_wiris_plugin_impl_HttpImpl), Array, Object(haxe_io_BytesOutput), true, 'Http Error #404')
#5 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/php/Boot.class.php(769): call_user_func_array('haxe_Http_5', Array)
#6 [internal function]: _hx_lambda->execute('Http Error #404')
#7 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/com/wiris/plugin/impl/HttpImpl.class.php(27): call_user_func_array(Array, Array)
#8 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/haxe/Http.class.php(444): com_wiris_plugin_impl_HttpImpl->onError('Http Error #404')
#9 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/haxe/Http.class.php(458): haxe_Http->customRequest(true, Object(haxe_io_BytesOutput), NULL, NULL)
#10 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/com/wiris/plugin/impl/HttpImpl.class.php(40): haxe_Http->request(true)
#11 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/com/wiris/plugin/impl/TextServiceImpl.class.php(80): com_wiris_plugin_impl_HttpImpl->request(true)
#12 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/service.php(19): com_wiris_plugin_impl_TextServiceImpl->service('mathml2accessib...', Array)
#13 {main}</pre>](/application/zrc/images/qvar/CHEN11086518.png)
since in
Arrange the molecules H2, O2, F2 and N2 in order of increasing bond length.
Write the MO electronic configuration or diatomic molecule having a bond order of 3.
The Molecular orbital configuration of N2;

Write the electronic configuration of 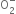 ion and predict its magnetic behaviour.
ion and predict its magnetic behaviour.
It is paramagnetic due to the presence of one unpaired electron.
Write the electronic configuration of  ion and predict its magnetic behaviour.
ion and predict its magnetic behaviour.
It is paramagnetic due to the presence of one unpaired electron.
Arrange the following molecular species in increasing order of stability:![]()
Order of increasing stability is given as,
Explain why H-bonding does not exist in HCl, though chlorine is quite electronegative.
Define vander Waal's forces.
Is hydrogen bond weaker or stronger than the vander Waal’s forces?
Why HF has higher boiling point than HCl?
Define chemical bond. Why do atoms combine (Kossel-Lewis approach) and how do atoms combine (modes of chemical combination)?
Chemical bond. A chemical bond may be defined as the attractive force or binding force which holds atoms, ions and molecules together.
Why do atoms combine (Kossel-Lewis approach)?
The noble gases are highly unreactive and stable. Except for He (1s2), all other noble gas elements have ns2npb outermost electronic configurations. This indicates that the presence of 8 electrons (law of octet) in the outermost orbit must be related to the stability of the atom. If K-orbit is the outermost orbit, the presence of 2 electrons (law of duplet) causes stability.
Thus atoms of different elements combine with each other in order to complete their respective octets (i.e. 8 electrons in their outermost shell) or duplet (i.e. outermost shell having 2 electrons) in the case of H, Li and Be to attain stable inert gas configuration.
How do atoms combine (Modes of chemical combination):
Atoms combine together in two ways to acquire stable inert gas configuration:
(i) By complete transference of one or more electron from one atom to another: This process is referred to as electro-valency and the chemical bond formed is termed as an electrovalent bond or ionic bond.
(ii) By sharing of elements: This can occur in two ways:
(a) When the shared electrons are contributed by the two combining atoms equally, the bond formed is called the covalent bond.
(b) When these electrons are contributed entirely by one of the atoms but shared by both, the bond formed is known as coordinated bond or dative bond.
What are Lewis symbols?
What is Lewis structure of an atom and anion?
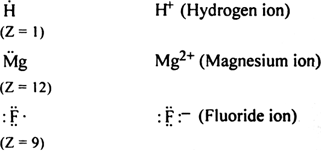
Sponsor Area
Write Lewis symbols for the following atom and ions:
S and S2-; Al and Al3+; H and H-.
The Lewis symbol for the given atom and ion is as,
S = 2, 8 and 6
(Z = 16)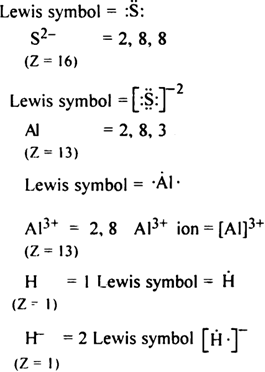
What is the significance of Lewis symbol? How do these help in finding the valency of an element?
(i) The symbol of an atom or ion stands for the nucleus as well as the electrons in the inner enthalpy shells.
(ii) The valence electrons are represented by dots near the symbol of the element.
Calculation of valency: For elements containing up to four valence electrons, the valency is usually equal to the number of valence electrons. For elements containing more than four electrons, the valency is either equal to the number of valence electrons or eight minus the number of valence electrons. For example.
The valencies of Li, Be, B and C = 1, 2, 3 and 4 (No. of valence electrons.
The valencies of N, O and F = 3, 2 and 1 (8 – valence electrons).
What is an ionic bond? How is it formed? Illustrate with suitable examples.
Ionic or electrovalent bond: The electrostatic force of attraction between the oppositely charged ions is known as an ionic bond. It is formed by the complete transference of one or more valence electrons of one atom to the valence shell of the other atom so that each atom acquires the nearest noble gas configuration. The compounds containing ionic or electrovalent bonds are called ionic or electrovalent compounds. The number of electrons which an atom loses or gains while forming an ionic bond is known as electrovalency. Examples :
(i) Formation of NaCl: The electronic configuration of sodium (Z= 11) and chlorine (Z= 17) can be represented as:.![]()
Thus, one electron gets transferred from Na atom to chlorine atom. This gives rise to Na+ and Cl– ions having a noble gas configuration. These ions are held together by the electrostatic force of attraction known as an ionic bond.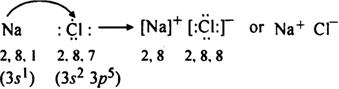
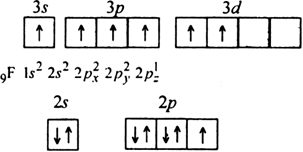
(ii) Formation of CaF2. The electronic configuration of calcium (Z = 20) and fluorine (Z = 9) can be represented as,
20Ca = 1s2 2s2 2p6 3s2 3p6 4s2 (2, 8, 8, 2)
9F = 1s2 2s2 2p5 (2, 7)
Thus, calcium atom loses two valence electrons to two fluorine atoms each of which gains one electron. This leads to the formation of Ca2+ ion and two F“ ions each of which has a stable noble gas configuration. These oppositely charged ions are mutually attracted by the electrostatic force of attraction which constitute ionic bonds.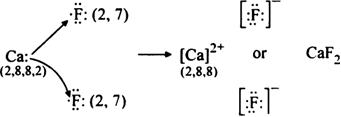
(iii) Formation of MgO: The electronic configuration of magnesium (Z = 12) and oxygen (Z = 8) can be represented as:
12Mg= 1s2 2s2 2p6 3s2 (2, 8, 2)
8O = 1s2 2s2 2p4 (2, 6)
Magnesium atom loses two valence electrons to an oxygen atom. Thus, Mg2+ and O2– ions are formed each of which has a stable noble gas configuration. These ions are held together by the electostatic force of attraction which constitutes ionic bond.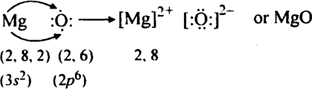
Draw the Lewis structures for the following molecules and ions:![]()
Lewis structure of the given molecule and ions are,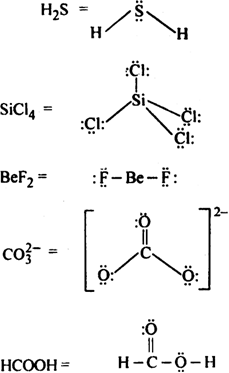
Use Lewis symbols to show electron transfer between the following atoms to form cations and anions:
(a) K and S, (b) Ca and O (c) Al and N
(a) K and S
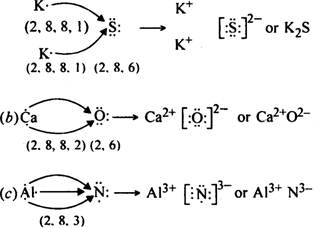
Discuss the factors which favour the formation of ionic bond.
Following factors influence the formation of ionic bonds:
(i) Low ionisation enthalpy: One of the atoms forming cation should have low ionisation enthalpy. Alkali metals (Group 1) and alkaline earth metals (Group 2) having low values of ionisation enthalpy from their cations readily.
(ii) Very high negative electron gain enthalpy: The other atom-forming anion should have very high negative electron gain enthalpy. Elements of group 16 and group 17 (halogens) having very high negative electron gain enthalpy from their anions readily.
M(g) → M+(g) + e– : Ionisation enthalpy
X(g) + e– → X –(g) : Electron gain enthalpy
M+(g) + X –(g)→ MX(s)
Clearly, ionic bonds will be formed more easily between elements with comparatively low ionisation enthalpies and elements with comparatively very high negative electron gain enthalpies.
Define lattice enthalpy. How is it violated to the stability of an ionic compound?
The greater the lattice enthalpy, more stable is the ionic compound.
What are important consequences of lattice enthalpies?
The consequences of lattice enthalpies:
(i) The greater the lattice enthalpy, more stable is the ionic compound.
(ii) The lattice enthalpy is greater, for ions of higher charge and smaller radii.
(iii) The lattice enthalpies effect the solubilities of ionic compounds.
Write three favourable factors for the formation of ionic bond ?
Three favourable factors for the formation of ionic bond are:
(i) Low ionisation enthalpy of the metal atom.
(ii) High electron gain enthalpy of the non- metal atom.
(iii) High lattice enthalpy of the compound formed.
What are important characteristics of ionic compounds?
(i) Stable existence: Ionic compounds usually exist in the form of crystalline solids. The crystals are made up of crystal lattices containing oppositely charged ions (+ve and –ve). Each cation is surrounded by a definite number of anions and vice-versa.
(ii) High melting and boiling points and low volatility: There is a strong force of attraction among the oppositely charged ions in the crystals of ionic compounds, so a large amount of enthalpy is needed to separate them. Due to these strong forces of attraction, ionic compounds have high melting and boiling points and low volatility.
(iii) Electrical conductivity: Ionic compounds do not conduct electricity in the solid state because oppositely charged ions occupy fixed positions in the crystals and are not free to move. When these crystals are dissolved in a polar solvent or melted, the ions can move freely under the influence of electric field and become conductor of electricity.
(iv) Fast reactions: The chemical reactions between ionic compounds involve the combination between the ions liberated in their aqueous solutions. So their reactions are very fast.
(v) Solubility: Ionic compounds are soluble in polar solvents (e.g. water) and insoluble in nonpolar (organic) solvents. Polar solvents have high values of a dielectric constant which reduces the electrostatic force of attraction between the oppositely charged ions. Hence, the ions get separated and ultimately solvated by the molecules of the polar solvent.
Can sodium chloride conduct electric current in the solid state?
No, sodium chloride can not conduct electricity in the solid state. This is because oppositely charged ions (Na+ and Cl– ions) are held together by a strong electrostatic force of attraction. These ions occupy fixed positions in the crystals and do not move when an electric field is applied.
An element A has the configuration 1s22s22p63s1 while the configuration of B is 1s22s22p5. What type of bond is likely to be formed between them?
Discuss the Lewis concept of the covalent bond formation.
A chemical bond formed by the mutual sharing of one or more pairs of electrons between atoms of same or different elements so as to complete their octets is called the covalent bond. Each atom contributes an equal number of electrons for the process of sharing and number of electrons contributed by each atom is known as covalency.
A single bond is formed when atoms mutually share one pair of electrons. A double bond is formed when the atoms share two electron pairs. A triple bond is formed when the atoms share three electron pairs.
Examples:
(a) Homoatomic molecules (covalent bonds in similar atoms).
(i) Hydrogen molecule (H2): A covalent bond is formed between two hydrogen atoms by sharing a pair of electrons between them. Each atom contributes one electron for sharing.![]()
(ii) Chlorine molecule (Cl2): Chlorine atom (17Cl) has seven electrons in its valence shell (2, 8, 7). In the formation of chlorine molecule, each chlorine atom contributes one electron for sharing in order to acquire a stable noble gas configuration (Argon).![]()
(iii) An oxygen molecule (O2): Oxygen atom (8O) has six electrons in its valence shell (2, 6). In the formation of O2 molecule, each oxygen atom contributes two electrons for mutual sharing in order to acquire a stable neon configuration and forms divalent or double bond.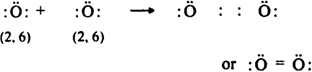
(iv) Nitrogen molecule (N2): Nitrogen atom (7N) has five electrons in its valence shell (2, 5). In the formation of N2 molecule, each nitrogen atom contributes three electrons for mutual sharing in order to acquire stable neon configuration and forms trivalent or triple bond.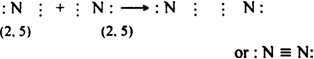
(b) Heteroatomic molecules (covalent bonds in different atoms).
(i) Methane molecule (CH4): Carbon atom (6C) has four electrons in its valence shell (2, 4). Carbon atom forms four covalent bonds with four hydrogen atoms.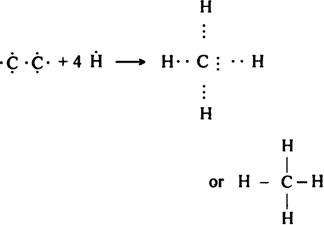
(ii) A water molecule (H2O): The oxygen atom is bonded to two hydrogen atoms.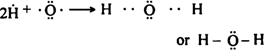
(iii) Carbon dioxide molecule (CO2): Carbon atom is bonded to two oxygen atoms with double bonds.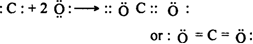
Write the Lewis dot structure of molecule.
Step I: The outer valence shell configuration of carbon and oxygen atoms are:
Total number of valence electrons = 4 + 6 = 10
Step II. The skeletal structure of Carbon and Oxygen is CO.
Step III. Putting one shared pair of electrons between C and O and completing the octet on O, the remaining two electrons are the lone pair on C.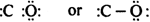
Step IV: In the above structure, octet of C is not complete. Hence multiple bonding is required between C and O. Octets of C and O will be complete if there is a triple bond between C and O.![]()
Write the Lewis structure of nitrite 

Step II. Total number of electrons to be distributed in

Step III. The skeletal structure of
 is O N O step.
is O N O step.Step IV. Putting one shared electron pair between the nitrogen and each of the oxygen atoms completing the octet of oxygen atoms.
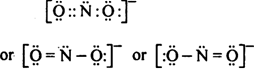
What do you understand by formal charge (F.C.) on atom in a molecule/ion?
The formal charge of an atom in a polyatomic molecule or ion may be defined as the difference between the number of valence electrons of that atom in an isolated or free state and the number of electrons assigned to that atom in the Lewis structure.
It is expressed as:
[Formal charge (F.C.) on an atom in a molecule/ion]
= [Total number of valence electrons in the free atom] - [Total number of non-bonding (lonepair) electrons]
- ![]()
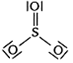
Calculate the formal chrage on each O-atom of O3 molecule.
The Lewis structure of O3 may be drawn as:

The atoms have been numbered as 1, 2 and 3. Formal charge (F.C.) on end O–atom numbered 1.
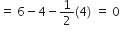
Formal charge (F.C.)on central O-atom numbered.
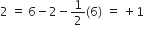
Formal charge (F.C.) on end O-atom numbered 3.
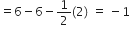
Hence, we represent O3 along with the formal charges as follows:
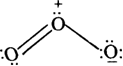
What is the importance of calculating the formal charges?
The main advantage of calculation of formal charges is that it helps to select the lowest energy structure (i.e. most stable structure) from a number of possible Lewis structures for a given species. The most stable are the one which has the smallest formal charges on the atoms.
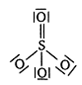
Calculate the FormalCharge on atoms in carbonate ion ?
Lewis structure of  ion is
ion is 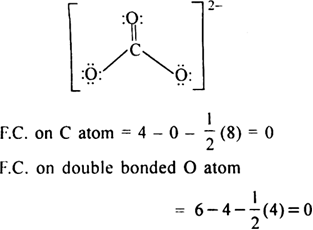
Formal Charge on single bonded O atom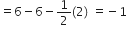
What do you understand by bond pairs and lone pairs of electrons? Illustrate by giving one example of each type.
What are the drawbacks of Lewis concept of bonding?
Lewis concept fails to explain:
(i) The cause of covalent bond formation.
(ii) The nature of attractive forces between the constituent atoms of a molecule.
(iii) The geometry of molecules containing covalent bonds.
(iv) The formation of molecules such as PCl5, SF6 and IF7 in which the central atom has more than 8 electrons in its valence shell (violation of octet rule).
(v) The formation of molecules such as BF3, AlCl3 in which the central atom has less than 8 electrons in its valence shell.
(vi) The amount of enthalpy released during covalent bond formation.
Discuss the formation of compounds in which octet rule is violated.
(i) Formation of compounds like BeCl2, BF3 AlCl3 etc. In each of these molecules, the central atom (Be, B or Al) has less than 8 electrons i.e. these are electron deficient compounds.
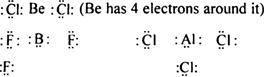
(B and Al have 6 electrons around them.)
(ii) Formation of compounds like PCl3, SF6, IF7 etc. In each of these molecules, the central atom has more than 8 electrons.

The octet rule is violated in these molecules.
Discuss the Modern(or Quantum) Theory of covalent bond.
Every system in this universe lends to acquire a state of minimum potential enthalpy in order to acquire maximum stability. Similarly, the formation of a bond between the atoms occur only it' there is a decrease in enthalpy.
The attractive forces tend to decrease the enthalpy of the system whereas repulsive forces tend to increase its enthalpy. When two atoms approach each other, the following two forces come into existence:
(i) Attractive forces between the electrons of one atom and the nucleus of the other atom.
(ii) Repulsive forces between electrons of the two atoms and also between their nuclei.
As a result of these electrostatic interactions, if there is a net decrease in enthalpy, a bond is formed between them. On the other hand, if there is an increase in enthalpy, the bond formation does not occur. For example, hydrogen molecule is formed but He2 is not formed.
Explain the formation of H2 molecule on basis of valence bond theory.
Or
In the light of attractive and repulsive forces, show that a molecule of hydrogen is formed.
Consider two hydrogen atoms A and B with electron eA and eB respectively. HArepresents the nucleus of hydrogen atom A and HB represents the nucleus of hydrogen atom B.
When the two hydrogen atoms approach each other, the following two forces come into existence:
(a) Attractive interactions in between:
(i) the nucleus HA an electron eB and
(ii) the nucleus HB and electron eA
(b) Repulsive interactions in between:
(i) electron eA and electron eB and
(ii) nucleus HA and nucleus HB.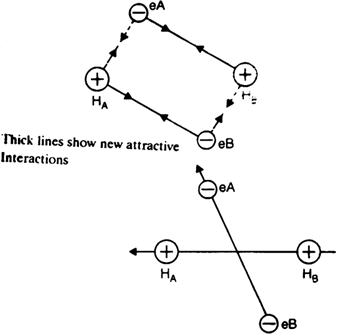
Since attractive forces overpower the repulsive forces, as a result, the enthalpy of the system decreases and a molecule of hydrogen is formed.
Enthalpy diagram: When two hydrogen atoms are at an infinite distance from each other, there is no interaction between them and therefore, the enthalpy of the system is assumed to be zero in this state (stage-A). As the two atoms start coming closer to each other, the potential enthalpy continues to decrease (stage B). Ultimately a stage is reached when the enthalpy of the system becomes minimum and hydrogen atoms are said to be bonded together to form a stable H2 molecule (state C).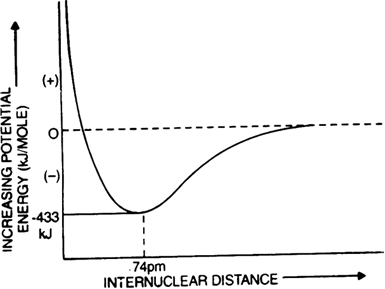
The internuclear distance r0 between two hydrogen atoms at this stage is referred to as bond length. In the case of the hydrogen molecule, the bond length is 74 pm. It should be noted that two hydrogen atoms can not be brought at a distance lesser than rQ (i.e. 74 pm) because the potential enthalpy of the system increases and curve shows an upward trend (dotted lines) and molecule becomes unstable.
In the light of attractive and repulsive forccs, show that helium molecule is not formed.
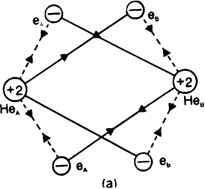
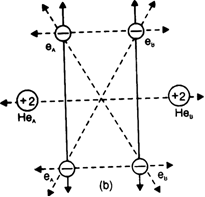
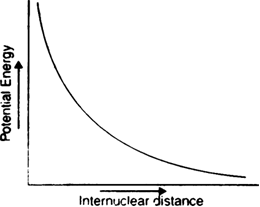
Thus energetically the formation of helium molecule is not possible because there is an increase in the potential enthalpy of the system when two helium atoms approach each other.
What are general characteristics of covalent compounds?
General characteristics of Covalent compound:
1. State of existence: Covalent compounds are generally gaseous or liquids. They may be solid (but soft) if the molecular mass is very high. Molecules are present in three states which are held together by a weak force called Vander Waal’s forces.
2. Low melting and boiling points : Covalent compounds have generally low melting and boiling points since only a little enthalpy is required to overcome the weak Vander Waal’s forces.
3. Electrical conductivity: They are generally non-conductors of electricity because they contain neither the ions for migration nor the free electrons.
4. Non-polar nature: They are generally non-polar or only slightly polar.
5. Solubility: Being non-polar or only slightly polar, they are generally insoluble in a polar solvent like water but soluble in non-polar solvents like ether, benzene etc.
6. Reactions (Non-ionic): Covalent compounds contain molecules and they undergo reactions slowly.
7. Nature of bond and isomerism: Covalent bond is rigid and directional (true bond). Hence covalent compounds exhibit various types of isomerism.
Give the comparison between electrovalent and covalent bonds.
| Electrovalent bonds | Covalent bonds |
| 1.Electrovalent compounds are formed by complete transfer of electrons in KCl (K. +.CI: — K+ Cl–) | 1. Covalent compounds are formed by mutual sharing of electrons as in NH3 |
| 2.Electrovalent compounds are made up of ions. | 2.Covalent compounds are made up of molecules. |
| 3.Electrovalent compounds are heard, crystalline solids e.g. NaCl, MgCl2 | 3. Covalent compounds are usually liquids or gases. E.g. CH4, C2H6, NH3 |
| 4. Electrovalent compounds are usually soluble in water but insoluble in non-polar solvents like Cl4 | 4.Covalent compounds are soluble in non-polar solvents like benzene or carbon tetrachloride and in soluble in polar solvents like water. |
| 5. Electrovalent compounds generally have high melting and boiling points. | 5.Covalent compounds generally have low melting and boiling points. |
| 6.Electrovalent compounds are good conductors of electricity in the molten state and in aqueous solutions but insulators in the solid state. |
6. Covalent compounds are bad conductors of electricity. |
Give the comparison between Electrovalent (or ionic compounds) compounds and covalent compounds (special reference to properties).
| Electrovalent compounds | Covalent bonds |
| 1. They are formed by the complete transference of one or more electrons from one atom to another. |
1.They are formed by the mutual sharing of electrons between two atoms. |
| 2. Exist as hard crystalline solids. | 2. Exist as gases, liquids or soft solids. |
| 3. Crystals are made up of ions. | 3. Crystals are made up of molecules. |
| 4. High melting and boiling points. | 4. Low melting and boiling points. |
| 5. It is a bad conductor of electricity in a solid state but conducts electricity in the molten state or in solution. | 5. Bad conductor of electricity |
| 6. Soluble in polar solvents, insoluble in non-polar solvents. | 6. Soluble in non-polar solvents, insoluble in polar solvents. |
| 7. Reactions are fast. | 7. Reactions are slow. |
| 8. Reactions are slow. | 8. Exhibit isomerism. |
Discuss the orbtial concept or quantum concept for the formation of covalent bond.
Or
Explain the formation of covalent bond on the basis of valence bond theory.
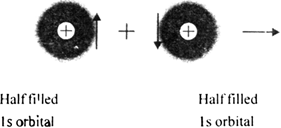

This results in the formation of molecular orbital which contains both the electrons which now have got paired.
What are the essential requirements for the formation of covalent bond?
The essential requirements for the formation of a covalent bond are,
(i) An atomic orbital of one atom containing one electron overlaps with an atomic orbital of other atom containing an electron of opposite spin.
(ii) Properly oriented atomic orbitals of different atoms can overlap together.
(iii) Greater the overlapping of orbitals, stronger will be the bond.
(iv) Overlapping of orbitals causes delocalization of electrons which in turn lowers the enthalpy and increases the stability.
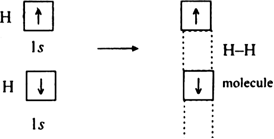
Discuss the orbital shapes of the following covalent molecules:
(i) H2 (ii) F2 (iii) O2 (iv) N2.
(ii) F2 molecule: The F2 molecule is formed by the overlapping of two half filled p-orbitals of two fluorine atoms. The electronic configuration of Fluorine is 1s2 2s2 2p5. Thus Overlapping of the orbital forms F2 molecule.

(iii) O2 molecule: The electronic configuration of oxygen (Z = 8) is

Thus oxygen atom has two half filled atomic orbitals. The oxygen molecule is formed when two half filled p-orbitals of each oxygen atom overlap with the two half filled p-orbitals of the other oxygen atom. This results in the formation of a double bond between two oxygen atoms (O = O).
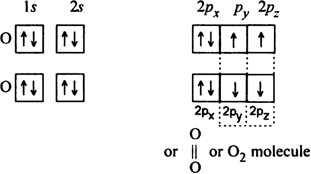
(iv) N2 molecule: The electronic configuration of nitrogen atom (Z = 7) is

The nitrogen atom has three half filled atomic orbitals. In the formation of N2 molecule, each of the three half, filled orbitals of one nitrogen atom overlaps with the three half filled orbitals of the other nitrogen atom.
This leads to the formation of a triple bond between two nitrogen atoms (N ≡ N).
Discuss the orbital shapes of the following covalent molecules:
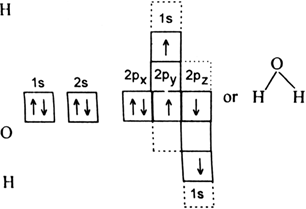
(i) HF (ii) H2O (iii) NH3.
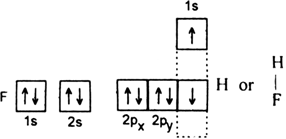
(ii) A water molecule (H2O): The electronic configuration of hydrogen is 1s1. Electronic configuration of oxygen 1s2 2s2 2p4. Therefore half-filled orbital of each hydrogen atom overlaps with each of the half-filled 2p–orbitals having electrons in opposite spins of oxygen atom to form H2O molecule.
(iii) Ammonia molecule (NH3): The electronic configuration of hydrogen is 1s1. Electronic configuration of nitrogen is 1s2 2s2 2p3. Therefore the orbital of each hydrogen atom overlaps with each of the three nitrogen orbitals.
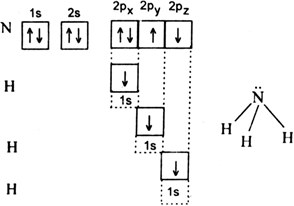
half-filled 2p orbitals of nitrogen having electrons in opposite spin to form NH3molecule.
Why is a sigma bond stronger than pi bond ?
A sigma bond stronger than a pi bond. The reason behind this is the orientation of the overlapped orbitals. Sigma bonds result from head-on(co-axial) overlapping while pi bonds are the outcome of lateral(para-axial) overlapping. Here is a pictorial representation of ethene(sp2 hybridised C atoms).
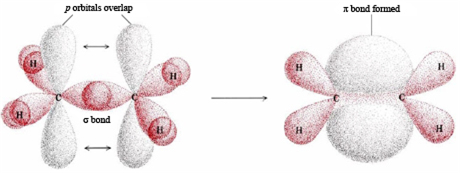
The greater the extent of overlapping, the higher the probability of finding the valence electrons in between the nuclei and hence the bond will be stronger.
Why are lone pair-lone pair repulsion stronger than lone pair-bond pair.
The lone pairs are localised on the central atom, while each bonded pair is shared between two atoms. consequently, the lone pair electrons in molecules occupy more space as compared to the bonding pair electrons. This causes greater repulsion between lone pairs of electrons as compared to the lone pair -bond pair and bond pair-bond pair repulsion.
Describe, in brief, the types of covalent bonds ?
A covalent bond is classified into two types:
1. Sigma (σ) covalent bond bond
2. pi (![]() ) covalent bond.
) covalent bond.
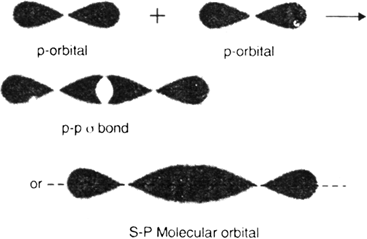
1. Sigma (σ) covalent bond (Axial overlap bond): A covalent bond formed by an axial (head on) overlapping of half filled atomic orbital is called sigma (σ) bond. The molecular orbital thus formed is symmetrical about the internuclear axis and is called sigma molecular orbital. Sigma bond may be formed by any one of the following types of overlapping: (i) s-s over-lapping (ii) s–p overlapping (iii) p-p overlapping half filled.
(s-s overlapping: It is the type of overlapping.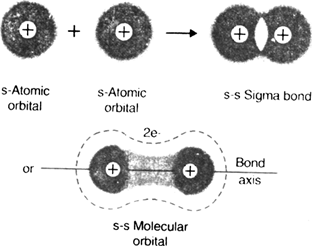
in which s-orbital of one atom overlaps with half filled s-orbital of another atom to form the s-s sigma bond. For example, in the formation of H2 molecule, Is orbitals of two hydrogen atoms mutually overlap along the internuclear axis to form a sigma bond.
(ii) s-p overlapping: It is the type of overlapping in which half filled s-orbital of one atom overlaps with half filled p-orbital of another atom to give s-p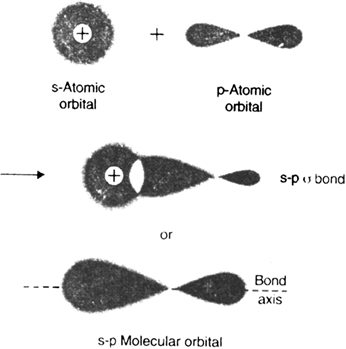
sigma bond. For example, in the formation of hydrogen fluoride molecule (H–F), half filled Is orbital of hydrogen atom overlaps coaxially with half-filled 2p–orbital of fluorine atom to form a sigma bond.
(iii) p-p overlapping: It is the type of overlapping in which half filled p-orbital of one atom overlaps with half filled p–orbital of another atom linearly i.e. along the axis (coaxial overlapping) to give p-p sigma bond. For example, in the formation of fluorine molecule (F – F), half-filled 2p–orbitals of two fluorine atoms mutually overlap coaxially to form a sigma bond.
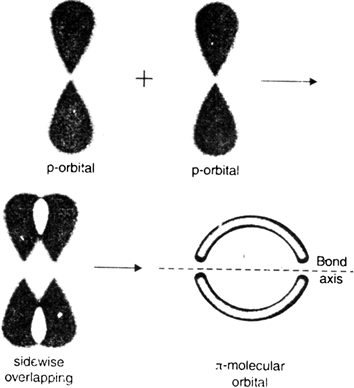
2.  A covalent bond formed by the sidewise or lateral overlapping (a non-axial overlapping) of two parallel oriented half filled p-orbitals is called
A covalent bond formed by the sidewise or lateral overlapping (a non-axial overlapping) of two parallel oriented half filled p-orbitals is called ![]() the bond. The electron density is localised above and below the plane of the bond axis.
the bond. The electron density is localised above and below the plane of the bond axis.
Using the orbital overlap concept, explain the formation of:
(i) O2 molecule (ii) N2 molecule.
(z = 8) is
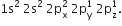
It has two half filled 2p-orbitals in its valence shell. In the formation of the oxygen molecule. One of the two half filled 2p-orbitals of each oxygen atom overlaps mutually along the internuclear axis to form σ bond. The other half-filled 2p orbitals of each oxygen atom undergo sidewise overlapping to form
Thus, two oxygen atoms are linked together by a double bond, one of which is sigma bond and other is

(ii) N2 molecule. The electronic configuration of nitrogen atom (Z = 7) is
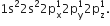 It has three half filled 2p-orbitals in its valence shell. In the formation of the nitrogen molecule, one of the three half-filled 2p orbitals of each nitrogen atom overlaps mutually along the internuclear axis to form a bond. The other two 2p-half filled orbitals of a nitrogen atom undergo sidewise overlapping with their parallel oriented 2p-orbitals of other nitrogen to form two
It has three half filled 2p-orbitals in its valence shell. In the formation of the nitrogen molecule, one of the three half-filled 2p orbitals of each nitrogen atom overlaps mutually along the internuclear axis to form a bond. The other two 2p-half filled orbitals of a nitrogen atom undergo sidewise overlapping with their parallel oriented 2p-orbitals of other nitrogen to form two 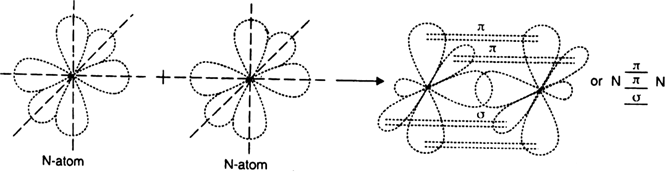
Distinguish between a sigma ![]() bond and a
bond and a ![]() bond.
bond.
| Sigma (σ) bond | pi |
| 1. It is formed by the coaxial over lapping of two half filled atomic orbitals along inter-nuclear axis | It is formed by the sidewise or lateral overlapping of two half filled p-orbitals perpendicular to the internuclear axis. |
| 2. This bond can be formed by overlap of s –s.s –p and p-p orbitals. | 2. It involves the overlap of p-orbitals only i.e. s orbitals can not participate in the formation of |
| 3. Sigma bond is stronger and less reactive. | 3. |
| 4. They have cylindrical symmetry of electron density about the bond axis. | 4. Electron density is localised above and below the plane of bond axis. |
| 5. Free rotation about a σ-bond is possible. | 5. Rotation of bond is restricted. |
| 6. Sigma bonds have independent existence. | 6. |
What do you mean by: (i) Bond length (ii) Bond enthalpy (iii) Bond order?
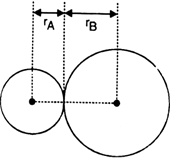
In the case of a covalent bond, the contribution from each atom is called covalent radius of that atom.
The bond length in a covalent molecule AB
R = rA + rB
where R is the bond length; rAand rB are the covalent radii of atoms A and B respectively.
(ii) Bond enthalpy: The strength of a chemical bond is measured as the bond dissociation enthalpy. It is the enthalpy required to break a particular bond in one mole of a gaseous molecule. For example for a homonuclear diatomic molecule H2, we have,
Similarly for a heteronuclear diatomic molecule HCl, we have,
![<pre>uncaught exception: <b>Http Error #404</b><br /><br />in file: /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/com/wiris/plugin/impl/HttpImpl.class.php line 61<br />#0 [internal function]: com_wiris_plugin_impl_HttpImpl_0(Object(com_wiris_plugin_impl_HttpImpl), NULL, 'http://www.wiri...', 'Http Error #404')
#1 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/php/Boot.class.php(769): call_user_func_array('com_wiris_plugi...', Array)
#2 [internal function]: _hx_lambda->execute('Http Error #404')
#3 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/haxe/Http.class.php(532): call_user_func_array(Array, Array)
#4 [internal function]: haxe_Http_5(true, Object(com_wiris_plugin_impl_HttpImpl), Object(com_wiris_plugin_impl_HttpImpl), Array, Object(haxe_io_BytesOutput), true, 'Http Error #404')
#5 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/php/Boot.class.php(769): call_user_func_array('haxe_Http_5', Array)
#6 [internal function]: _hx_lambda->execute('Http Error #404')
#7 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/com/wiris/plugin/impl/HttpImpl.class.php(27): call_user_func_array(Array, Array)
#8 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/haxe/Http.class.php(444): com_wiris_plugin_impl_HttpImpl->onError('Http Error #404')
#9 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/haxe/Http.class.php(458): haxe_Http->customRequest(true, Object(haxe_io_BytesOutput), NULL, NULL)
#10 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/com/wiris/plugin/impl/HttpImpl.class.php(40): haxe_Http->request(true)
#11 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/com/wiris/plugin/impl/TextServiceImpl.class.php(80): com_wiris_plugin_impl_HttpImpl->request(true)
#12 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/service.php(19): com_wiris_plugin_impl_TextServiceImpl->service('mathml2accessib...', Array)
#13 {main}</pre>](/application/zrc/images/qvar/CHEN11086585-2.png)
For molecules like O2 and N2 containing double and triple bonds:
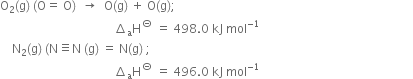
(iii) Bond Order: According to Lewis concept of a covalent bond, the bond order is given by the number of bonds between the two atoms in a molecule. For example,
Bond order in H2 (H – H) = 1
Bond order in O2 (O = O) = 2)
Bond order in N2 (N ≡ N) =3
Similarly, the bond order in CO (three shared electron pairs between C and O) is 3.
Isoelectronic molecules and ions have identical bond order; For example, F2 and O22–have bond order 1. N2, CO and NO+ have bond order 3.
It should be noted that with the increase in bond order, bond enthalpy increases and bond length decreases.
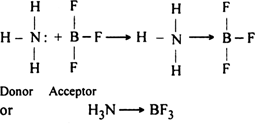
What is coordinate or dative bond? Explain the formation of the bond with some examples.
Coordinate bond: It is the type of bond in which electron pair for sharing is provided by only one of the two bonded atoms i.e. out of the two bonded atoms one with already completed octet provides its lone pair of electrons for sharing to the other bonding atom having a shortage of electrons. The atom which provides the electron pair for sharing is known as a donor and the other atom which accepts the electron pair is called acceptor. The bond is represented by an arrow→ pointing from donor towards the acceptor.
If the atom A has its octet already complete and the atom B has only six electrons in its valence shell, then a coordinate bond is formed.
![]()
Examples:
(i) A coordinate bond is formed between ammonia (NH3) and boron trifluoride (BF3).
(ii) Formation of  ion.
ion.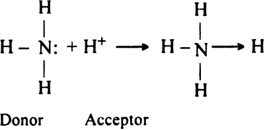
(iii) Formation of  ion:
ion: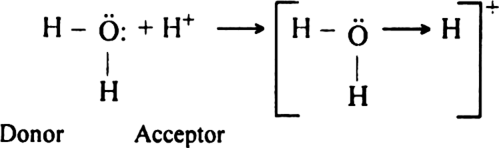
What are the conditions for co-ordinate bonding?
A covalent bond is formed by two atoms sharing a pair of electrons. The atoms are held together because the electron pair is attracted by both of the nuclei.
In the formation of a simple covalent bond, each atom supplies one electron to the bond.
The electronegativity of the atoms should not be high with respect to each other.
What are physical important characteristics of coordinate compounds?
The physical important characteristics for coordinate compounds are:
(i) Physical state: They may be gaseous, liquids or solids.
(ii) Melting and boiling points: They generally have low melting and boiling points. However, their melting and boiling points are relatively more than the covalent compounds.
(iii) Conductivity: Generally, they are poor conductors of electricity but some of them are good conductors also.
(iv) Solubility: Generally, they are insoluble in water but soluble in organic solvents. However, some of them are soluble in water.
(v) Isomerism: They exhibit stereoisomerism due to rigid and directional nature of the coordinate bond.
Explain coordinate bond by the orbital concept.
A coordinate bond is formed by the overlapping of an orbital of an atom containing a lone pair of electrons with the empty orbital of the other atom.
Let us consider the formation of ammonium ion (N+H4). It is formed by the combination of ammonia molecule and hydrogen ion.
The central nitrogen atom of ammonia molecule has one orbital containing a lone pair of electrons while the other three orbitals are filled up with bond pairs of electrons.
On the other hand, hydrogen ion has empty is orbital. When the H+ ion approaches ammonia molecule, its empty orbital overlaps the orbital of nitrogen atom containing a lone pair to form a dative bond. 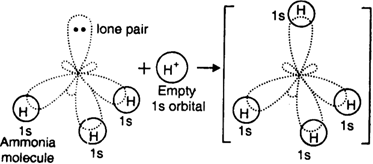
Differentiate between a covalent bond and co-ordinate bond.
| Covalent Bond | Co-ordinate Bond |
| 1. This is formed by sharing of two electrons. Each atom supplies electron. A. +. B → A:B. |
1. One atom contributes an electron pair while the sharing is done by both atoms. X : + Y → X : Y |
| 2. Denoted by A - B | 2. Denoted by Xδ+ → Yδ– |
| 3. Formed between electron deficient atoms. | 3. Formed between a donor with a lone pair and an acceptor (electron pair deficient). |
| 4. Formed between atoms. | 4. Formed between molecules or between molecules and ions. |
What do you understand by geometry and shapes of molecules?
Why do covalent moleucles have definite geometery?
As a result:
(i) All bonded atoms occupy such positions (around the central atom) in space where the repulsive forces between them are minimum.
(ii) The molecule attains minimum enthalpy and maximum stability.So molecule has a definite shape or geometry.
Name the theory responsible for the definite geometry of covalent molecules. Give main features of the theory ?
The theory responsible for the definite geometry of covalent molecules is called valence shell electron pair repulsion theory (VSEPR theory).
The main points of this theory are:
1. The geometry of a molecule depends upon the number of electron pairs (bonded pairs as well as non bonded) surrounding the central atom.
2. The electron pairs surrounding the central atom repel each other because their electron clouds are negatively charged. As a result, electron pairs try to stay as far away as possible to attain a state of minimum enthalpy or maximum stability.
3. The relative magnitudes of the relative interactions between pair of electrons on the central atom are as follows:
Lone pair-Lone pair (lp | lp) > Lone pair-Bond pair (lp | bp) > Bond pair-Bond pair (bp | bp).
4. Repulsive forces decrease sharply with increasing angle between the electron pairs. They are strong at 90°, weaker at 120° and much weaker at 180°.
Different geometries of molecules depending upon the number of electron pairs in the valence shell of the central atom are summed up as:
| No. of electron pairs around central atom | Geometric arrangement | Bond angles | Examples |
| 1 | Linear | 180° | BeF2, ZnCl2, HgCl2 |
| 2 | Trigonal Planar | 120° | BF3, AlCl3 |
| 3 | Tetrahedral | 109.5° | CH4,CCl4 |
| 4 | Trigonal | 120° | SiCl4 |
| 5 | Bipyramidal | 90° | PF5, PCl5 |
| 6 | Octahedral | 90° | SF6, TeF6 |
What do you mean by Regular geometry and Irregular or Distorted geometry?
Regular geometry: The molecules in which the central atom is surrounded only by similarly bonded electron pairs will have regular geometries. The central atom should have no lone pair of electrons and should be bonded to all similar atoms. For example CH4, CCl4, BF3 etc.
Irregular or Distorted geometry: The molecules in which the central atom is surrounded by bond pairs, as well as lone pairs, will have irregular geometries. The central atom may be bonded to similar atoms but should have different bond lengths.
For example, CHCl3, CHBr3, PF5.
How will you explain the following order of the repulsive interactions between pair of electrons on the central atom:
Lone pair - Lone pair > Lone pair - Bond pair > Bond pair - Bond pair.
The lone pair are localised on the central atom, each bonded pair is shared between two atoms. As a result, the lone pair electrons in a molecule occupy more space as compared to the bonding pairs of electrons. This result in greater repulsion between the lone pairs of electrons as compared to the Lone pair -bond pair and bond pair-bond pair repulsion.
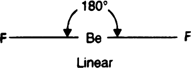
On the basis of VSEPR theory, discuss the geometry of the following covalent molecules: (i) BeF2 (ii) BF3 (iii) CH4.
To have a minimum repulsion in the electron pairs around the central atom, the geometry of the molecules is regular as well as linear. Bond angle in such cases is 180°.
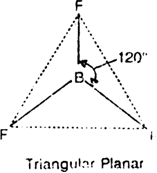
(iii) BF3: The electronic configuration of B (Z = 5) is 1s2 2s2 2p1x. The central atom B has 3 valence electrons. These three valence electrons are shared mutually with the electrons of the fluorine atoms to form three B - F bonds.
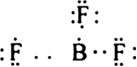
Thus, B atom is surrounded by three bond pairs. These repel each other and go as far apart as possible so that there are no further repulsions. This is so if the three electron pairs are placed at 120° with respect to each other i.e. the most favourable arrangement is the triangular planar geometry.
(iii) CH4 The electronic configuration of C(Z =6) is
The central atom has 4 valence electrons. These four valence electrons are shared mutually with the electrons of four hydrogen atoms to form four C - H bonds as shown.
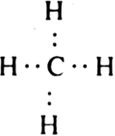
Thus carbon atom is surrounded by four bond pairs. In order to minimise the inter-electron pair repulsions i.e. having a state of minimum enthalpy and hence maximum stability, these bond pairs should be as far apart from one another as possible. This is so if the four electron.pairs are placed at an angle of 109.5° with respect to each other i.e. the most favourable arrangement is tetrahedral geometry. In other words, carbon is present in the centre of a regular tetrahedron and four bond pairs are directed to the four corners with bond angle-critical to 109.5°.
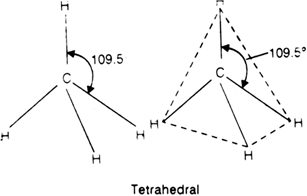
With the help of VSEPR theory, explain the shape of: (i) NH3 (ii) H2O.
 has five electrons in the valence shell. Three of these electrons are mutually shared with the electrons of three hydrogen atoms to form three N- H bonds as shown.
has five electrons in the valence shell. Three of these electrons are mutually shared with the electrons of three hydrogen atoms to form three N- H bonds as shown. 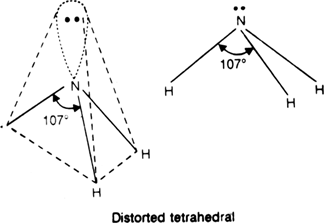
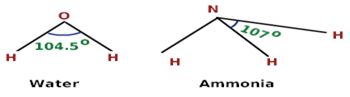
Hence, the central N atom in NH3 is surrounded by three bond pairs and one lone pair. The geometry expected for the molecule is tetrahedral since lone pair-bond pair repulsion is more than bond pair-bond pair repulsion. As a result, the lone pair of electrons will repel another pair strongly. Therefore three N–H bonds of NH3 are forced slightly closer.
This leads to decrease in H – N – H bond angles from a normal angle of a tetrahedron (109.5°) to 107°. The most favourable arrangement is distorted tetrahedral i.e. pyramidal. In this, nitrogen atom lies at the centre and three hydrogen atoms occupying the triangular base and the orbital with a lone pair of electrons from the apex of the pyramid.
(ii) H2O: In a water molecule, the central oxygen atom
 has six electrons in the valence shell. Two of these electrons are mutually shared with the electrons of two hydrogen atoms to form two O - H bonds.
has six electrons in the valence shell. Two of these electrons are mutually shared with the electrons of two hydrogen atoms to form two O - H bonds. Hence, the central oxygen atom is H2O is surrounded by two bond pairs and two lone pairs. These four electron pairs adopt tetrahedral arrangement. The presence of two lone pairs brings

distortion in the geometry of the molecule. The lone pairs repel the bond pairs more effectively resulting in the decrease of H – O – H angle from 109.5° to 104.5°. The water molecule may be regarded as bent or angular or V-shaped.
Although geometries of NH3 and H2O molecules are distorted tetrahedral, the bond angle in water is less than that of ammonia. Discuss.
On the basis of VSEPR theory, predict the shapes of given molecules: PCl5 and SF6.
(i) PCl5 molecule. The electronic configuration of central phosphorus atom is![]()
It has five valence electrons. All the five electrons are mutually shared with the electrons of five chlorine atoms to form five P - Cl bonds as shown.
Hence, P atom is surrounded by five shared pairs of electrons. These repel each other and take up such positions and there is no further repulsion between them. The most favourable arrangement is trigonal bipyramidal.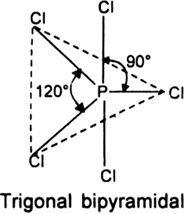
(ii) SF6 molecule: The electronic configuration of central sulphur atom is![]()
It has six valence electrons.
All the six valence electrons are mutually shared by the electrons of six fluorine atoms to form six S – F bonds.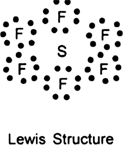
Hence, S atom is surrounded by six shared pairs of electrons (six bond pairs). These repel each other and try to remain as far apart as possible so that there is no further repulsion between them. Under such conditions, the most favourable arrangement is octahedral.
Discuss the shape of the following molecules using VSEPR model:
BeCl2, BCl3, SiCl4, AsF5, H2S, PH3
BeCl2:
Lewis dot structure Cl: Be : Cl. The central atom (Be) has only two bond pairs and no lone pair. Hence shape is linear.![]()
BCl3:![]()
The central atom (B) has only three bond pairs and no lone pair. Hence shape is triangular planar.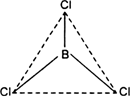
SiCl4:![]()
The central atom (Si) has four bond pairs and no lone pair. Hence the shape is tetrahedral.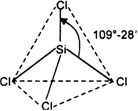
AsF5:![]()
The central atoms (As) has five bond pairs and no lon∈ pair. Hence, the shape is trigonal bipyramidal.
H2S:![]()
The central atom (S) has two bond pairs and two lone pairs. Hence, the shape is Bent or V-shaped.
PH3:![]()
The central atom (P) has three bond pairs and two lone pairs. Hence, the shape is. Bent or V-shaped.

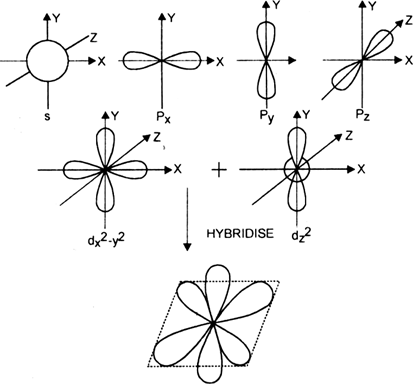
State and explain hybridisation.
The phenomenon of intermixing of atomic orbitals of slightly different enthalpies of an atom so as to redistribute their enthalpies to form the same number of new orbitals of equivalent enthalpies and identical shapes is called hybridization. The new orbitals, thus formed are called hybrid orbitals or hybridised orbitals.
Explanation : In order to understand hybridisation, let us take an example of carbon (Z= 6). Its ground state electronic configuration is,![]()
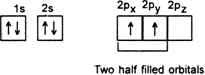
Since it has two half filled orbitals, therefore, the valency of the carbon atom should be 2. But actually, carbon atom always exhibits a valency of four (tetravalent). To achieve this, an electron is promoted from 2s filled orbital to the vacant higher enthalpy 2p orbital. This is called excited state of a carbon atom.

In the excited state of carbon s and p, orbitals have different enthalpies. Consequently, four bonds of carbon must be of two types. Three of the bonds should be of one type (s - p bonds) while fourth bond should be a different type (s - s bond). However, experimental evidence indicates that all the four bonds in case of CH4 (methane) are equivalent. To explain the equivalence of all the four bonds in case of methane, the concept of hybridisation is used i.e. all the four orbitals in the valence shell of carbon may get mixed, redistribute enthalpies and give orbitals of new enthalpy and shape. These equivalent orbitals are called hybrid orbitals.
What are the necessary conditions for hybridisation?
The necessary conditions for hybridization:
(i) The orbitals taking part in hybridization must have only a small difference of enthalpies.
(ii) The orbitals undergoing hybridisation generally belong to the valence of the atom.
(iii) It can take place between completely filled, half-filled or empty orbitals.
(iv) All the orbitals of the valence shell may or may not take part in hybridisation.
What are the different types of hybridisation in s and p atomic orbitals?
These are of three types:
(i) sp type (Linear)
(ii) sp2 type (Trigonal)
(ii) sp3 type (Tetrahedral).
Discuss in brief sp3 hybridisation. Explain the formation of methane and ethane.
This type of hybridization involves the mixing of all four filled orbitals i.e. 1s and 3p orbitals to form four new orbitals called sp3 hybrid orbitals of equivalent enthalpies and identical shapes.
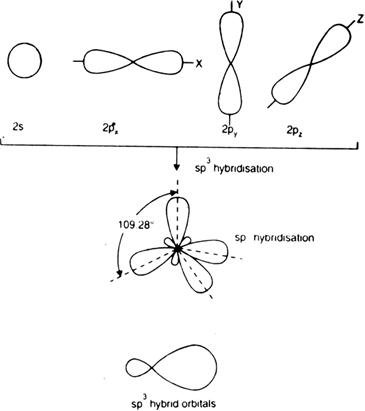
Fig. Representation of sp3 hybridization:
A single sp3 hybrid orbital.
The four sp3 hybrid orbitals are directed towards the four corners of a tetrahedral. The angle between two adjacent sp3 hybrid orbitals. Each sp3 hybrid orbital has 1/4 s-character and 3/4 p-character. sp3 hybridization is also known as tetrahedral hybridisation.
(i) The molecular orbital structure of methane: In methane molecule, carbon atom undergoes sp3 hybridisation. Each sp3 hybrid orbital overlaps with 1s orbital of hydrogen atom along the internuclear axis to form four σ bond.
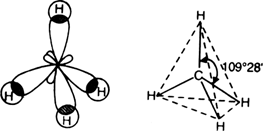
Molecular orbital structure of methane
The four C – H bonds are directed towards the four corners of a regular tetrahedron. So methane has a tetrahedral structure. Each H – C – H bond angle is of 109°.28’. Each C – H bond length is 109 pm (1.09 Å).
(ii) Molecular orbital picture of ethane: In ethane molecule, both carbon atoms are in the sp3 hybrid state. In its formation, one hybrid orbital of one carbon atom overlaps with one sp3 hybrid orbital of a second carbon atom along the internuclear axis to form a sigma (σ) C – C bond. The remaining three sp3 hybrid orbitals of each carbon atom overlap with 1 s orbital of hydrogen atom axially to form six sigma C – H bonds.
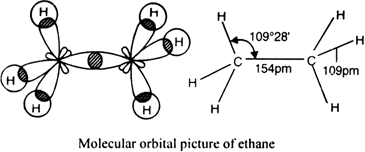
The length of C-C bond in ethane is 154 pm (or 1- 54 Å) and that of each C - H bond is 109 pm (or 1-09 Å).
Discuss in brief sp2 hybridization (hybridization in C = C bond). Discuss the molecular orbital structure of ethylene (first member of alkene).
Or
Draw diagrams showing the formation of a double bond between carbon atoms in C2H4.
 .
.In this type of hybridization one- s and two P-orbitals of the valence shell of carbon atom take part in hybridization go give three new sp2 hybrid orbitals. These sp2 hybrid orbitals lie in a plane and are directed towards the corners of an equilateral triangle with a carbon atom in the centre. The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals.

Representation of sp2 hybridization sp2 hybridization is also known as trigonal hybridisation. Each sp1 hybrid orbital has

The molecular orbital structure of ethylene: In ethene molecule, each carbon atom undergoes sp2 hybridisation. One sp2 hybrid orbital of one carbon atom overlaps axially with one sp2 hybrid orbital of the other carbon atom to form sigma (σ) C - C bond. The other two sp2 hybrid orbitals of each carbon atom overlap axially with its orbital of the hydrogen atom to form sigma (σ) C - H bonds. The unhybridized p-orbitals of the two carbon atoms overlap sidewise with each other to form weak pi (

(a) Formation of ethylene (b) Molecular orbital structure molecule of ethylene
Thus, ethylene molecule consists of four sigma C – H bonds, one sigma C - C bond and one
Apart from tetrahedral geometry, another possible geometry for CH4 is square planar with four H atoms at the corners of the square and the C atom at its centre. Explain why CH4 is not square planar?
Here 1 s orbital & 3 p orbitals undergo hybridization to form sp3 hybrid orbitals .Hence, carbon atom undergoes sp3 hybridization in CH4 molecule and takes a tetrahedral shape.
For a square planar shape, the hybridization of the central atom has to be dsp2, ie, 1 s orbital, 3p orbitals & 1 d orbitals have to undergo hybridization .However, an atom of carbon does not have d-orbitals to undergo dsp2 hybridization. Hence, the structure of CH4 cannot be square planar.
Moreover, with a bond angle of 90° in square planar, the stability of CH4 will be very less because of the repulsion existing between the bond pairs. Hence, VSEPR theory also supports a tetrahedral structure for CH4.
On the basis of hybridisation, discuss the orbital structure of: (i) BeF2 (ii) BH3.

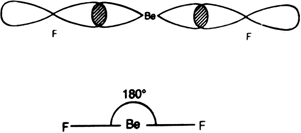
To form BeF2 molecule, an electron gets promoted from filled 2s orbital to vacant 2p orbital. Therefore, the excited configuration of Be atom is
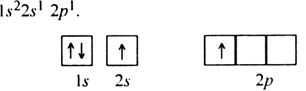
In BeF2, Be atom is sp hybridised i.e. two hybrid orbitals are directed along a straight line with a bond angle equal to 180°. Each sp hybrid orbital overlaps axially with 2p half-filled orbital of F atom to form sigma Be – F bonds.
(ii) Orbital structure of BH3:
The electronic configuration of B (Z = 5) is 1s2 2s22P1.

In BH3, B atom is sp2 hybridised and the three hybrid orbitals overlap axially with the orbital of the hydrogen atom to from three sigma B–H bonds.

The shape is trigonal planar.
The central atoms in CH4, NH3 and H2O are all said to have similar hybridisation but the bond angle H – A – H (where A is C, N or O) is different in each case. Explain stating in which case it is maximum and in which case it is minimum.

Discuss in brief the various types of hybridisation involving d-orbitals.
The elements present in the third period contain a-orbitals in addition to s and p orbitals.
The energy of the 3d orbitals is comparable to,
(i) the energy of 3s and 3p orbitals
(ii) the energy of 4s and 4p orbitals.
Hence the hybridization involving either 3s, 3p and 3d or 3d, 4s and 4p is possible. However, since the difference in energies of 3p and 4s orbitals is significant, therefore no hybridization involving 3p, 3d and 4s orbitals is possible.
The hybridisation schemes involving s, p and d orbitals are summarised below:
| Shape of molecules/ions | Hybridisation type | Atomic obritals | Examples |
| Squre planar | dsp2- | d+s+p(2) |
[Ni(CN)4]2–, [Pt(Cl)4]2– |
| Trigonal Bipyramdial | sp3d | s+p(3)+d | PF5.PCl5 |
| Square pyramidal | dsp3 | d+s+p( 3) | BrF5,XeOF4 |
| Octahedral | sp3d2 | s+p(3)+d(2) | SF6,[CrF6]3– |
| d2 sp3 | d(2)+s+p(3) |
[Co(NH3)6]3+ |
State and explain the geometric arrangements possible in sp3d and sp3d2 hybridisation. Name the d-orbitals involved in these.
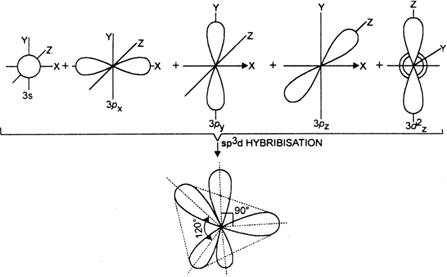
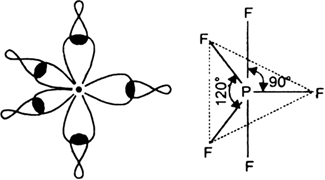
In this type, one s, three p and one d-orbital (of a central atom of a compound) of nearly the same energy intermix to give five equivalent sp3d hybrid orbitals. These hybrid orbitals point towards the five corners of a trigonal bipyramid. Out of these five sp3d hybrid orbitals, three are directed towards the three corners of an equilateral triangle while the remaining two are at right angles to the plane of the first three orbitals, d-orbitals, involved in this case is dz2 For example in PF5, sp3d hybridization is involved.
sp3 d2 hybridisation:
In this type, one s, three p and two d atomic orbitals of approximately same energy intermix to give six equivalent sp3d2hybrid orbitals which are directed towards six corners of a regular octahedron. It should be noted that the orbitals (s, p, d) which are mixed together belong to the same quantum shell (n). The two d orbitals involved in this hybridization are dx2–y2 and d2 Shapes of participating orbitals are as shown, e.g. in SF6, sp3 d2 hybridization is involved.
On the basis of hybridisation, explain the shape of phosphorus penta fluoride (PF5).
It is the case of sp3d hybridization in which one 3s, three 3p and one 3d orbital of P-atom having nearly same energy intermix to given five sp3 dihybrid orbitals. Now five hybrid orbitals are available for a combination which permits the formation of five covalent bonds with five fluorine atoms of PF5

But all the hybridised orbitals are not equivalent. Three of these are oriented towards the three corners of an equilateral triangle making an angle of 120° between them. The bonds formed by these orbitals are known as equatorial bonds. Remaining two orbitals are oriented at right angles to the plane of the first set of three orbitals. Thus PF5 has trigonal bipyramidal shape.
Discuss the shape of:

on the basis of hybridization.
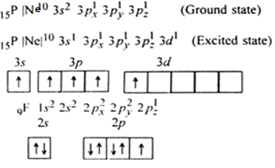
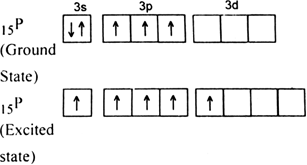
In PCl5 , the central atom is 15P. The electronic configuration of phosphorus and chlorine atoms are as follows:
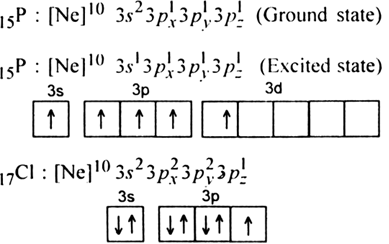
It is the case of sp3d hybridisation in which one 3s, three 3p and one 3d orbital of P-atom having nearly same energy intermix to give five sp3d hybrid orbitals. Now five hybrid orbitals are available for a combination which permits the formation of five covalent bonds with five chlorine atoms of PCl5
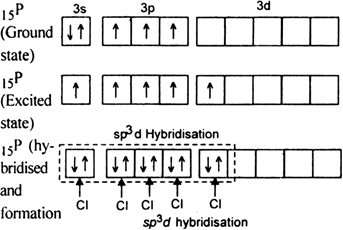
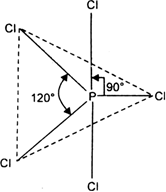
But all the hybridised orbitals are not equivalent. Three of these are oriented towards the three corners of an equilateral triangle making an angle of 120° between them. The bonds formed by there orbitals are known as equatorial bonds. Remaining two orbitals are oriented at right angles to the plane of the first set of three orbitals. Thus PCl5 has trigonal bipyramidal shape.
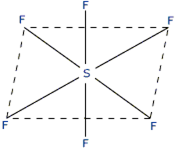
(ii) The shape of SF6: In SF6, the central atom is 16S. The electronic configuration of sulphur and fluorine atoms are as follows:
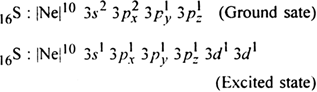
It is the case of sp3d2 hybridisation in which one 3s, three 3p and two 3d orbitals of S atom having nearly same energy intermix to give six sp3d2 hybrid orbitals. Now six hybrid orbitals are half filled.
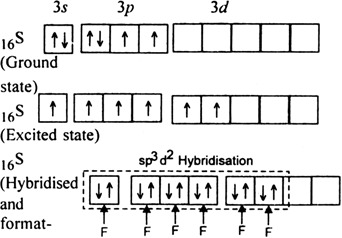
Half filled 2p orbitals of six F atoms overlap with six-half filled sp3d2 hybrid orbitals of S-atom to form six sp3d2 p-p sigma bonds. So SF6 molecule has an octahedral structure.
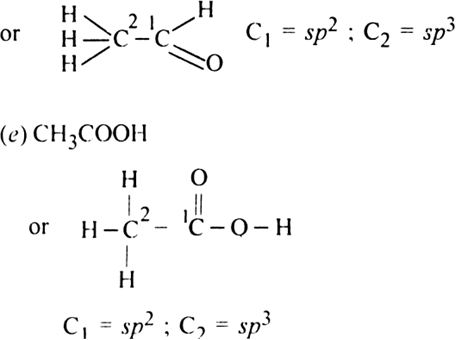
Which hybrid orbitals are used by carbon atoms in the following molecules ?
(a) CH3 – CH3
(b) CH3 – CH = CH2
(c) CH3 – CH2 - OH
(d) CH3 - CHO
(e) CH3COOH
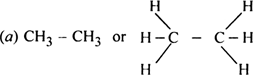
Both carbon atoms use sp3 hybrid orbitals.
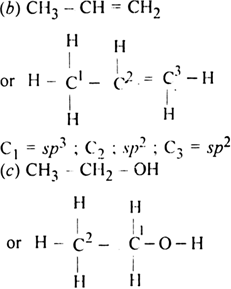
Both carbon atoms (C1, C2) use a sp3 hybrid orbital.
(d) CH3CHO
Describe the change in hybridization (if any) of the Al atom in the following reaction:![]()
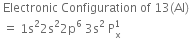
13Al (Excited state) = ls22s-22p63s13px1 3py1 .Hence in AlCl3, Al undergoes sp2 hybridization to give it planar triangular structure. In AlCl4 , the empty 3p. orbital is also involved so that hybridization is sp3 and its shape is tetrahedral.
Is there any change in the hybridisation of B and N atoms as a result of the following reaction?
BF3 + NH3 → F3B.NH3?

What are non-polar and polar covalent bonds? Give examples.
(ii) Polar covalent bond. If a covalent bond is formed between two dissimilar (non-identical) atoms having different electronegativities, the shared pair of electrons is pulled closer towards more electronegative atom, such a bond is called the polar covalent bond. The more electronegative atom acquires partial negative charge (δ–) while less electronegative atom acquires a partial positive charge (δ+). For example, hydrogen fluoride molecule has a polar covalent bond.
Other molecules having polar covalent are H-Cl, H2O and NH3.
Can a non-polar molecule have polar covalent bonds? Explain.
Yes, it is possible in case of the linear molecule. For example, carbon dioxide is non-polar although both carbon-oxygen bonds in carbon dioxide are polar. Also, symmetrical molecules such as methane and carbon tetrachloride are non-polar although four carbon-chlorine bonds in CCl4 are polar because polarities of the bonds mutually cancel out and molecules are of non-polar nature.
Why does chlorine form more polar hydride than iodine?
Chlorine is non-polar molecule while HCl is polar covalent, Explain?
Hence, chlorine is a non-polar molecule. On the other hand, in H-Cl the bonding electrons are not equally shared by the two atoms. The shared electron pair is strongly attracted towards the more electronegative chlorine atom.
Anionic bond is an extreme case of a polar covalent bond. Justify.
What causes the two atoms to combine to form a bond which is:
(i) Non-polar
(ii) Polar-covalent and
(iii) Ionic in nature?
The nature of the bond between the two atoms depends on upon the electronegativity difference in them.
(i) If the two atoms are identical or have the same electronegativity (C and S), the bond is of non-polar nature.
(ii) If the two atoms differ in electronegativity, but the difference is less than 1 -9, the bond is mainly polar covalent with little ionic character. For example, the bond between carbon and chlorine.
(iii) If the difference in electronegativity of two atoms is more than 1 -9, the percentage ionic character is more than the percentage polar covalent character. Larger the electronegativity difference, greater will be the ionic character. For example, the bond between sodium and fluorine is completely ionic.
Briefly explain the term 'Dipole moment'. How is it expressed and what are its units?
Hδ+ - Clδ–.
The polarity of a molecule is expressed in terms of dipole moment. It is the product of the magnitude of the charge on either end of the dipole and the distance separating the charges. If e is the magnitude of the charge and d is the distance separating the charges, then
μ = e X d
where μ represents the dipole moment.
Dipole moment is a measure of the polarity of the covalent bond. It is expressed by an arrow pointing from positive pole to the negative pole with a small tail at the positive charge (→).
Unit of dipole moment. Since the charge is of the order of 10–10 e.s.u. and distance is of the order of 10–8 cm, the value of the dipole moment is of the order of 10–10 - 10–8 e.s.u. cm or 10–18 e.s.u. cm = 1
Debye or 1 D.
∴ μ = 10–18 e.s.u. cm = i D
Thus, units of dipole moment are debye (D).
Although both CO2 and H2O are triatomic molecules, the shape of H2O molecule is bent While that of CO2 is linear. Explain this on the basis of dipole moment.
The CO2 molecule has a zero dipole moment even though C and O have different electronegativities and each of the C = O bond is polar and has the same dipole moment. This indicates that the individual dipole moments are equal in magnitude and pointed in opposite directions and as a result, they cancel out each other.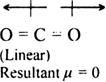
On the other hand, water is a polar molecule having a dipole moment, 1.84D. It is because water has been a bent structure in which two O-H bonds are oriented at an angle of 104.5° and do not cancel the dipole moments of each other. The molecular dipole moment of water (μ =1.84 D) is the resultant of the individual values of the dipole moment of two O – H bonds.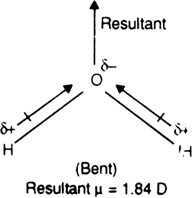
Explain why BeH2 molecule has a zero dipole moment although the Be-H bonds are polar?
Carbon tetrachloride and ammonia have dipole moment values. Explain.

On the other hand, ammonia is a polar molecule having resultant dipole moment 1•49 D.
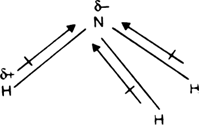
The molecular dipole moment of ammonia molecule is the resultant of three polar N – H bonds oriented in space at an angle of 107° with respect to each other.
Both BF3 and CH4 have zero dipole moment. Explain.

On the other hand, methane molecule has zero dipole moment because the individual dipole moments cancel out because of the symmetrical tetrahedral shape of the molecule.

Sketch the bond moments and resultant dipole moment in SO2, cis and trans forms of CH2Cl2.
SO2: SO2 molecule has a bent shape in which two S = O bonds are oriented at an angle of 119-5°. The bond dipoles and the resultant dipole moment in the SO2 molecule are represented as shown.
Cis form of CH2Cl2 has two chlorine atoms on the same side has a small resultant dipole moment while trans– form of CH2Cl2 has two chlorine atoms on the opposite side, the bond dipoles of the two C – Cl bonds cancel each other.
Therefore the resultant dipole moment of trans form is zero.
The dipole moment of hydrogen halides decreases from HF to HI. Explain this trend.
In H-X, X (F,Cl, Br, I) atom is more electronegative than H. Therefore,the H-X bond is polar and the direction of the dipole is from H to X. As the electronegativity of X decreases from F to I the charge separation decreases. Therefore, the dipole moment decreases.
For example, the dipole moment of hydrogen halides follow the order:
H - F > H - Cl > H -Br > H - I
Electro- 1 - 9 1-1 0-9 0- 6 ‘ negativity difference.
Dipole- 1-91 1-07 0-79 0-38 moment (D).
Which out of the two molecules - OCS and CS2 has a higher dipole moment and why ?
Discuss the applications (or importance) of dipole moment.
(i) The degree of polarity in a molecule : In , the case of diatomic molecules, the greater the polarity of the molecule, greater is the dipole moment. For example, HF (μ = 1 . 91 D) is more polar than HCl (μ= I -03 D).
(ii) The distinction between polar and non-polar molecules: Non-polar molecules like H2, CO2,CCl4 etc. have zero dipole moment whereas polar molecules such as HCi, NH3 and H2O etc. have some value of dipole moment. The non-polar molecules may have polar or non-polar bonds.
(iii) Shapes of molecules. The value of dipole moment provides useful information about the shapes of molecules. For example, triatomic molecules having zero dipole moment have linear shape. For example, CO2, CS2 etc., while those triatomic molecules which have some dipole moment have angular shape. For example, H2O (μ =1.84) has an angular shape.
The molecule of SO2 has a dipole moment. Is the molecule linear or bent? Explain your answer.
The linear molecules have zero dipole moment because in such molecules the polarities of the bonds cancel out each other.
Predict the dipole moment of;
(i) a molecule of the type AX4 having a square planar geometry.
(ii) a molecule of the type AX5 having a trigonal bipyramdial.
(iii) a molecule of the type AX6 having an octahedral geometry.
(i) It has zero dipole moment.
(ii) It has specific dipole moment.
(iii) It has zero dipole moment.
Which of the two - NH3 and NF3 has higher dipole moment and why?
NH3 has a higher dipole moment. Both these molecules have the pyramidal shape with one lone pair.
In the case of NH3, the orbital dipole due to lone pair is in the same direction as the resultant dipole moment of the three N – H bonds. Therefore, it adds on the resultant of the N – H bonds. But in a case of NF3, the orbital dipole due to the lone pair is in the opposite direction to the resultant dipole moment of the three N – F bonds. Thus the lone pair moment cancels the resultant N – F bond moment. Consequently, the dipole moment of NF3 is low.

What do you understand by resonance?
(i) Benzene molecule is a resonance hybrid of the following two main contributing structures:

(ii) SO2 is a hybrid of the following two contributing structures:
Discuss the resonance in CO2 and O3molecule.

The above different contributing structures of CO2 molecule differ only in the distribution of electrons and not in the arrangement of atoms. Thus, C to O bond has a single bond, double bond as well as a triple bond character since all the structures contribute towards the hybrid.
Resonance in (Ozone). The structure of ozone molecule is angular. Ozone molecule is the resonance hybrid of the following two structures:
These two contributing structures (i) and (ii) of O3 molecule differ only in the distribution of electrons.
Write the resonance structures for SO3, NO2 and ![]()
Resonance structure of the given molecule,
(i) SO3 :
Write the resonance structure for:
(i) Benzene (C6H6)
(ii) Carbonate ion (CO3–)
Resonance structure for the given molecule ism,
(i) Benzene (C6H6):
What is resonance enthalpy? What is its significance?
Resonance enthalpy = Enthalpy of the most stable contributing structure - enthalpy of resonance hybrid.
Due to the low fo resonance hybrid, it is more stable than any of its contributing structures. In other words, it can be said that resonance hybrid is always more stable because of resonance enthalpy. Larger the resonance enthalpy, more will be the stability of the compound. Also, relative stabilities of two hybrids can be compared in terms of resonance enthalpies.
Define moleuclar orbital. What are the salient features of Molecular orbital theory?
A molecular orbital may be defined as a region in space associated with the nuclei of bonded atoms in a molecule where the probability of finding a particular electron is maximum.
Salient features:
(i) Molecular orbitals are formed by the linear combination of atomic orbitals having nearly the same energies.
(ii) Molecular orbitals are associated with the nuclei of the bonded atom in a molecule.
(iii) The number of molecular orbitals formed is equal to the number of combining atomic orbitals.
(iv) When two atomic orbitals combine, they form two new orbitals called bonding molecular orbital and anti-bonding molecular orbital. The bonding molecular orbital has lower energy whereas anti-bonding molecular orbital has higher energy than the atomic orbitals.
(v) The shapes of the molecular orbitals formed depend on upon the type of the combining atomic orbitals.
(vi) The bonding molecular orbitals are represented as σ,![]() δ etc. whereas the corresponding antibonding molecular orbitals are represented as
δ etc. whereas the corresponding antibonding molecular orbitals are represented as ![]() etc.
etc.
(vii) The filling of the molecular orbitals takes place according to the same rule as those of atomic orbitals. These are:
(a) Molecular orbitals are filled in order of their increasing energies (Aufbau principle).
(b) Molecular orbital can have maximum two electrons and these must have opposite spin (Pauli’s exclusion principle).
(c) A pairing of electrons in the degenerate molecular orbitals does not take place until each of them has one electron each (Hund’s rule of maximum multiplicity).
Give at least two differences between atomic and molecular orbitals.
| Atomic orbitals | Molecular orbitals |
| 1. The electron cloud extends around the nucleus of a single atom. | 1. The electron cloud extends around all the nuclei of bonded atoms in the molecules. It is obtained by combining atomic orbitals of comparable energy. |
| 2. They are less stable. | 2. They are more stable. |
| 3. They have a simple shape. | 3. They have a complex shape. They are represented by |
| 4. They are represented by s,p,d,f etc. | 4. They may be bonding or antibonding. |
Give three points of difference between hybrid orbitals and molecular orbitals ?
| Hybrid orbitals | Molecular orbitals |
| 1. Valence atomic orbitals are involved. | 1. All orbitals are involved. |
| 2. Atomic orbitals of one atom are used in the formation of hybrid orbitals. | 2. Atomic orbitals of two or more atoms are involved in the formation of molecular orbitals. |
| 3. Hybrid orbitals are of equal energy. | 3. Molecular orbitals are of different energies. |
What is a linear combination of atomic orbitals (LCAO) far obtaining molecular orbitals ?
Molecular orbitals are formed by the combination of atomic orbitals. On combining two atomic orbitals, we get two molecular orbitals; one molecular orbital with lower energy is called bonding molecular orbital and its wave function is obtained by the linear addition of wave function and ΨA and ΨB of bonded atoms A and B.
ΨMO = ΨA + ΨB (Bonding MO)
The other molecular orbital with higher energy is called antibonding molecular orbital and its wave function is obtained by linear subtraction of wave functions of ΨA and Ψ B of atomic orbitals.
ΨMO = ΨA – ΨB (Antibonding MO).
Give the points of difference betweeen bonding and antibonding molecular orbitals.
Differences between bonding and antibonding orbitals.
| Bonding molecular orbital | Antibonding molecular orbital |
| 1. It is formed by the addition overlap of atomic orbitals. Ψ MO = ΨA + ΨB |
1. It is formed by the subtraction overlap of atomic orbitals. Ψ MO = ΨA – ΨB |
| 2. It possesses lower energy than the atomic orbitals from which it is formed. | 2. It possesses higher energy than the atomic orbitals from which it is formed. |
| 3. There is a greater electron density between the nuclei of bonded atoms. | 3. There is less electron density between the nuclei of interacting atoms. |
| 4. Every electron in a bonding molecular orbital contributes to the attraction between two atoms. | 4. Every electron in an antibonding molecular orbital contributes to repulsion between two atoms. |
| 5. These are designed as σ, |
5. These are designated as |
Write the important conditions required for the linear combination of atomic orbitals to form molecular orbitals.
The following are the conditions for the linear combination of atomic orbitals:
(i) The combining atomic orbitals should have comparable energies.
(ii) The combining atomic orbitals must have proper orientations (same symmetry) so that they are able to overlap to a considerable extent. For example, assuming 2-axis as molecular (or internuclear axis), allowed the combination of atomic orbitals are 2pz orbital of one atom can combine with 2pz orbital of another atom. On the other hand, 2pz orbital of one atom cannot combine with 2px or 2py orbital of another atom or 2px can not combine with 2py. Similarly, 2s orbital of one atom can combine with 2pz orbital but cannot combine with 2lpx or 2py orbital of another atom because they do not have proper orientation for the overlap.
(iii) The extent of overlapping should be large. Greater the overlap, greater will be the electron density.
Write the significance of a plus and minus sign shown in representing the orbitals.
The orbital is the maximum probability of finding an electron around the nucleus. This probability is measured in terms of the wave function. The wave function can be positive or negative values.
Since orbitals are represented by wave functions, therefore a plus sign in an orbital represents a positive wave function and a minus sign represents a negative wave function. Further the wave functions of two 1s atomic orbitals can combine in two different ways:
(i) When both have the same signs.
(ii) When they have different signs.
Considering x–axis as the internuclear axis which out of the following will not form a sigma bond and why? (a) 1 s and 1 s (b) 1 s and 2px : (c) 2py and 2py (d) 1s and 2s.
Assuming Z-axis as molecular (or internuclear) axis, label the molecular orbtials formed by the following combinations of atomic orbitals: (i) 1 s + 1 s (ii) 2py – 2pv (iii) 2Pz – 2pz.
(i) Combination of 1s and 1s atomic orbitals gives rise two molecular orbital such as bonding and antibonding : 
(ii) Combination of 2py and 2py atomic orbitals gives rise to give two molecular orbitals, such as bonding and antibonding: 
(iii) The combination of 2pz and 2pz atomic orbitals gives rise to give two molecular orbitals such as bonding and antibonding molecular orbital.
Discuss the shapes of molecular orbitals formed by the combination of the following atomic orbitals. (lateral overlap)
(i)2px + 2py (ii) 2pz – 2pz

(ii) 2p7 – 2pz: The two atomic orbitals combine by subtraction. The molecular orbital formed by the subtraction overlap of 2pz orbitals along the internuclear axis is designed as antibonding molecular orbital called o2pz orbital.

Which of the following combinations of orbtials are allowed in LCAO method (considering z-axis to be molecular axis) and sketch the shapes of molecular orbitals formed by their addition and subtraction:
(i) s and pz
(ii) px and px
(iii) pz and py
(iv) s and px.
Allowed combinations:
(i) s and p.z combination
(ii) px and px combination.
Not allowed combinations:
(iii) py and py combination Since they have
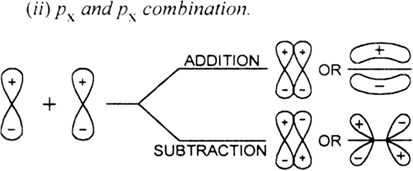
Give the points of difference between sigma and pi molecular orbitals ?
| σ-Molecular orbital | |
| 1. It is formed by the overlap of atomic orbitals along the internuclear axis. | 1. It is formed by the sidewise (lateral) overlapping of the atomic orbitals. |
| 2. Due to head on the overlap, the overlapping is maximum | 2. Due to sidewise overlap, overlapping is minimum. |
| 3. It consists of one electron cloud. | 3. It consists of two electron clouds, one lying above and the other lying below a plane passing through the nuclei. |
| 4. Its electron cloud is symmetrical about the internuclear axis. | 4. Its electron cloud is not symmetrical about the internuclear axis. |
What informations are conveyed from the electronic configuration of the molecule?
The information is conveyed from the electronic configuration.
(a) From the electronic configuration, it is possible to find out the number of electrons in bonding molecular orbitals (Nb) and a number of electrons in anti-bonding orbitals (Na).
(i) The molecule is stable if Nb > Na.
(ii) The molecule is unstable if Nb < Na.
(iii) The molecule is unstable if Nb = Na.
It may be noted that even if the number of electrons in bonding M.O. and the number of electrons in anti-bonding M.O. are equal, the atoms do not combine to form a molecule. This is because of the fact that the destabilising effect of anti– bonding electrons is slightly more than the stabilising effect of bonding electrons.
(b) To predict the magnetic behaviour of molecules i.e. if all the electrons in a molecule are paired, the substance is diamagnetic and in case there are unpaired electrons in a molecule, the substance is paramagnetic.
Define bond order. What informations are conveyed by bond order?
Bond order may be defined as half the difference between number of electrons in bonding molecular orbitals and the number of electrons in antibonding molecular orbitals, i.e.,

Information conveyed by Bond Order:
(i) If the value of the bond order is positive, it indicates a stable molecule and if the value of the bond order is negative or zero, it means that the molecule is unstable and is not formed.
(ii) The dissociation energy of the molecule is directly proportional to the bond order of the molecule i.e. greater the bond order, greater is the bond dissociation energy.
(iii) Bond length of the molecule is inversely proportional to the bond order of the molecule i.e. greater the bond order, the shorter will be the bond length.
(iv) Knowing the bond order, the number of covalent bonds between the atoms in the molecule can be predicted. Bond order of a molecule is equal to the number of covalent bonds between the atoms in the molecule.
(v) If the bond order is fractional, the molecule will definitely be paramagnetic. However, if the bond order is the whole number, the molecule may or may not be paramagnetic.
Give the number of electrons which occupy the bonding molecular orbital in H2+ H2 and He2.
(i) The number of electrons in bonding molecular orbital of H2+ is 1.
(ii) The number of electrons in bonding molecular orbital of H is 2.
(iii) The number of electrons in bonding molecular orbital of He2 is 2.
What do you understand by bond order? How is it related with bond length and bond energy? Explain on the basis of bond order that He2 molecule does not exist.
Bond order is inversely proportional to bond length and directly proportional to bond energy.
Bond order of
Thus He2 consists of no bonds i.e. bond does not exist.
Why the bond orders of He2+ and H2– are identical?
Molecular ion Molecular orbital configuration
![]()
![]()
![]()
Since both He2+ and H2– have equal number of bonding (Nb) and antibonding (Na) electrons because of identical molecular orbital configuration, their bond order is given by 
Using LCAO method for the formation of molecular orbitals in case of homonuclear diatomic hydrogen molecule.
According to LCAO method, molecular orbitals are formed by the combination of atomic orbitals.
In the case of H2 molecule, each H-atom consists of one atomic orbital (i.e. 1s) having one electron each.
On combining these two atomic orbitals of two H-atoms, we get two molecular orbitals for H2 molecule. One molecular orbital is lower in energy called bonding molecular orbital and its wave function is obtained by linear addition of wave functions of two
H-atoms Ψ (1s) + Ψ Ψ (1s) → Ψ MO. (bonding MO called a is ).
The other molecular orbital with higher energy is called antibonding molecular orbital and its wave function is obtained by linear subtraction of wave functions of two H-atoms as:
Ψ (1s) – Ψ (1s) → Ψ MO
(Antibonding M.O. called).
The energy level diagram of H2 molecule can be given as: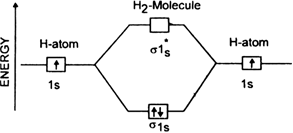
Draw the molecular orbital diagram for:
(i) Be2
(ii) B2 and predict bond order and magnetic properties.
(i) Be2 molecule: The electronic configuration of Be(Z = 4) is:
4 Be 1s2 2s1
Be2 molecule is formed by the overlap of atomic orbitals of both beryllium atoms.
Number of valence electrons in Be atom = 2
Thus in the formation of Be2 molecule, two outer electrons of each Be atom i.e. 4 in all, have to be accommodated in various molecular orbitals in the increasing order of their energies.
.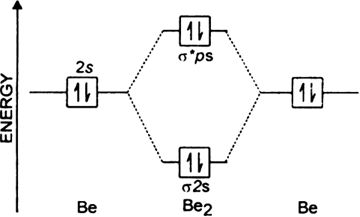
The molecular orbital electronic configuration,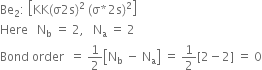
Magnetic property: Since bond order is zero, Be2 molecule does not exist. It is diamagnetic due to the absence of any unpaired electron.
B2 molecule: The electronic configuration of B atom (Z = 5) is![]()
B2 molecule is formed by the overlap of atomic orbitals of both boron atoms. A number of valence electrons of each boron atom = 3.
In the formation of B2 molecule, three valence electrons of each boron atom i.e. 6 in all, have to be accommodated in various molecular orbitals in the increasing order of their energies.
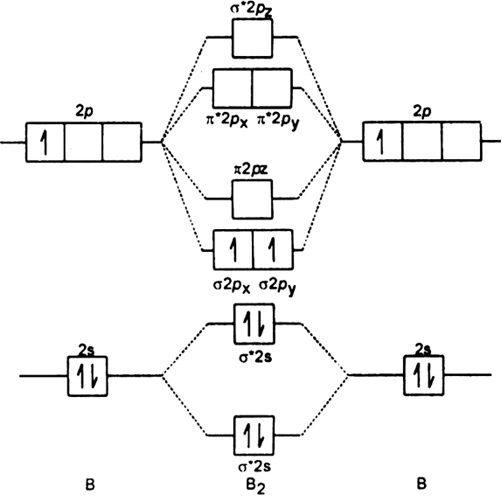
MO electronic configuration:![]()
Bond order: Here Nb = 4, Na = 2
Bond order = ![]()
The two boron atom is B2 molecules are linked by one covalent bond.
Magnetic properties: Since each ![]() 2px and
2px and ![]() 2py MO contains unpaired electron, therefore B2 molecule is paramagnetic.
2py MO contains unpaired electron, therefore B2 molecule is paramagnetic.
Is B2 molecule paramagnetic or diamagnetic? Explain.
The M.O. electronic configuration of B2 molecule is
B2: [KK (σ2s)2 (σ2s)2 (![]() 2px)1(
2px)1(![]() 2py)1]
2py)1]
Since each ![]() 2px and
2px and ![]() 2py MO contains unpaired electron, therefore B2 molecule is paramagnetic.
2py MO contains unpaired electron, therefore B2 molecule is paramagnetic.
Discuss the formation of N2 molecule on the basis of MO theory. Predict its:
(i) Bond order
(ii) Magnetic character.
The electronic structure of nitrogen atom is ![]() Leaving out 4 electrons in the 1s orbital of two nitrogen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 10 electrons in nitrogen (N2) is as shown as below:
Leaving out 4 electrons in the 1s orbital of two nitrogen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 10 electrons in nitrogen (N2) is as shown as below: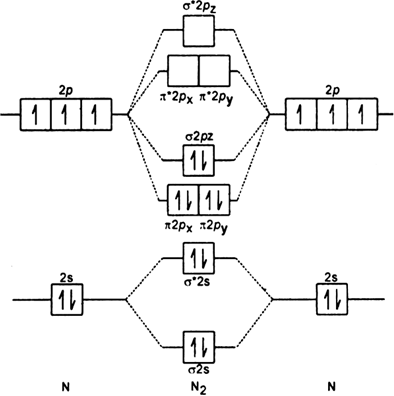
(i) Electronic configuration:
N2 : [KK (σ2s)2 (σ2s)2 (![]() 2px)1(σ2pz)2]
2px)1(σ2pz)2]
(i) Bond order : Here Nh = 8 and Na = 2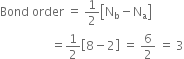
The two nitrogen atoms in nitrogen molecule are linked by three covalent bonds (i.e. a triple bond).
(ii) Magnetic character: Since all the electrons are paired, nitrogen is diamagnetic.
Write the stability configuration of ![]() and predict their bond order, stability and magnetic character.
and predict their bond order, stability and magnetic character.
a) ![]() ion: It is formed from
ion: It is formed from ![]() a molecule by the loss of one electron. This electron will be lost from 2p7 orbital of nitrogen molecule.
a molecule by the loss of one electron. This electron will be lost from 2p7 orbital of nitrogen molecule.
(i) Electronic configuration:
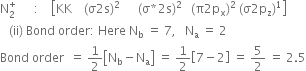
(iii) Stability: As the bond order is positive, it is quite stable.
(iv) Magnetic character: Since 2pz orbital has one unpaired electron, therefore it is paramagnetic.
(b) ![]() ion : It is formed by the gain of one electron by N2 molecule.
ion : It is formed by the gain of one electron by N2 molecule.
This electron will go to either ![]() orbital of N2 each of which is empty.
orbital of N2 each of which is empty.
(i) Electronic configuration.![]()
(ii) Bond order: Here Nb = 8; Na = 3![]()
(iii) Stability. Bond order being positive, N2 is quite stable.
(iv) Magnetic character Due to the presence of an unpaired electron in ![]() *2px orbital, it is paramagnetic.
*2px orbital, it is paramagnetic.
(c) ![]() ion: It is formed when molecule gains two electrons,
ion: It is formed when molecule gains two electrons,
(i) Electronic configuration![]()
(ii) Bond order: Here Nb = 8; Na = 4![]()
(iii) Stability. Since bond order is positive, it is quite stable.
(iv) Magnetic character: As it has two unpaired electrons one each in  orbitals, it is paramagnetic.
orbitals, it is paramagnetic.
Draw the molecular orbital energy diagram for oxygen molecule (O2) and show that:
(i) It has a double bond
(ii) It has paramagnetic character.
 Leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 12 electrons of oxygen as molecule is shown:
Leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 12 electrons of oxygen as molecule is shown: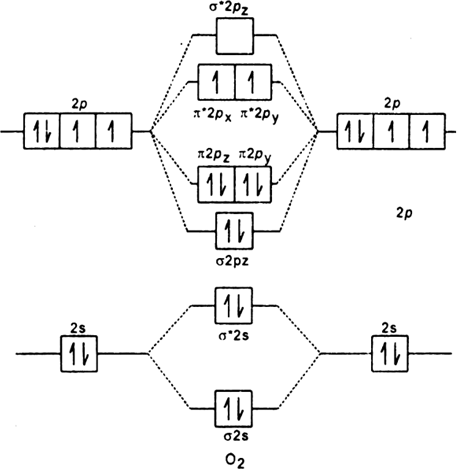
(i) Electronic configuration:
(ii) Bond order: Here Nb = 8; Na = 4
The two oxygen atoms in a molecule of oxygen are united through two covalent bonds (i.e. a double bond).
(iii) Paramagnetic character : Since a molecule of oxygen has two unpaired electrons in the
Using a molecular orbital diagram, predict the bond order, stability and magnetic character of O2–,O2+, and  . Also, write their electronic configurations.
. Also, write their electronic configurations.
O2–(Superoxide ion): This ion is formed by the addition of one electron.
O2 + e- → O2
This additional electron will be added up in the ![]() molecular orbital.
molecular orbital.
Electronic configuration:![]()
Bond order:
Here Nb = 8; Na = 5
Stability : As the bond order is positive, it is quite stable.
Magnetic character: It has one unpaired electron in the ![]() molecular orbital.
molecular orbital.
therefore, it is paramagnetic.
O+2 ion This ion is formed by the loss of one electron from O2 molecule.
O2 →O2+ e–
This electron will be lost from ![]() *2px or
*2px or ![]() *2py molecular orbital.
*2py molecular orbital.
Electronic configuration:![]()
Bond order: Here Nb = 8; Na = 3![]()
Stability: As the bond order is positive, it is quite stable.
Magnetic character: Since ![]() ion has one unpaired electron in the
ion has one unpaired electron in the ![]() orbital, therefore, it is paramagnetic.
orbital, therefore, it is paramagnetic. : The ion is formed when O2 molecule gains two electrons
: The ion is formed when O2 molecule gains two electrons
These two electrons will be added to the ![]() *2px and
*2px and ![]() *2py molecular orbitals, one in each.
*2py molecular orbitals, one in each.
Electronic configuration:
Bond order: Here Nb = 8; Na = 6![]()
Stability: As the bond order is positive, it is quite stable.
Magnetic character: As one of the molecular orbital has a unpaired electron, it is diamagnetic.
Discuss the relatives stabilities, bond dissociation energies and bond lengths of O2, 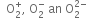 species.
species.
For O2 molecule:
Nb = 8, Na = 4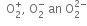
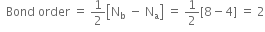
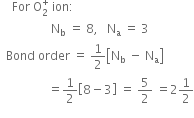



As bond dissociation energies are directly proportional to the bond order, therefore, the dissociation energies of these molecular species are in order: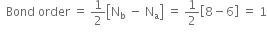
As greater the bond dissociation energy, greater is the stability; the stability of these species is also as in the above order.
As bond length is inversely proportional to bond order, therefore their bond lengths will be in order:
Is the bond order in superoxide ion more or less than in peroxide ion? Explain on the basis of MO theory.
The MO electronic configuration of superoxide ion ![]() is
is
Here ![]()

The M.O. electronic configuration of peroxide ion ![]() is
is ![]()
So, the bond order in superoxide ion is more than in peroxide ion.
Using bond order show that N2 would be expected to have a triple bond, F2 a single bond and Ne2 no bond.

Thus F2 consists of a single bond.
(iii) Bond order of Ne2
Thus Ne2 consists of no bonds.
Show that N2 molecule has a greater bond dissociation energy than N-2 where O2 has lower bond dissociation energy than O+2.
The bond dissociation energy is directly proportional to bond order such that,
greater the bond order, greater is the bond dissociation energy.

∴ N2 has greater bond dissociation energy than N-2. O2 has a lower bond dissociation energy than O+2.
Which of the following has higher bond dissociation energy and why?
(i) N+ (ii) O+

Since the bond order of both N2 and O2 is same, the stability is to be divided in terms of antibonding electrons. Since the last e- in O2 enters antibonding orbital, it is thermally less stable and has lesser bond dissociation energy.
Thus
Give the reason for the following: Bond order in N2 is 3 whereas it is 2.5 in NO ?
Total number of electrons in N2 = 7 + 7 = 14
The electronic configuration is:

Total number of electrons in NO = 7 + 8 = 15. The 15th electron enters into ![]()
Now Nb = 8 and Na = 3![]()
Give the points of resemblance and points of difference between valence bond theory and molecular orbtial theory.
Points of resemblance: According to both the theories:
(i) The bond results from the overlap of atomic orbitals.
(ii) The atomic orbitals overlapping each other must be the same energy and must have the same symmetry.
(iii) The electron charge resides in the region between the atomic nuclei.
(iv) Both are approximate theories.
(v) Account the directional character of covalent bonds.
(vi) Predict the non-existence of helium molecule (He2) and neon molecule (Ne2).
(b) Points of difference:
| Valence Bond theory | Molecular Orbital Theory |
| 1. Electrons in molecules are localised as if they are in isolated atoms. | 1. It treats the nuclei of the molecules as polycentric and then constructs the molecular orbitals. |
| 2. Atomic orbitals of participating atoms retain their individual character to a large extent. | 2. Atomic orbitals of the participating atom lose their individual character. |
| 3. It fails to explain paramagnetism of O2. | 3. It explains the paramagnetism of O2. |
| 4. Resonance is an important part of valence bond theory | 4. Resonance has no role in this theory. |
| 5. It is simple to apply. | 5. It is more difficult to apply. |
| 6. It fails to explain the possibility and existence of H2+ | 6. It explains the possibility and existence of H2+ |
What is hydrogen bond? How is it formed?
A hydrogen bond can be defined as the attractive force which binds hydrogen atom of one molecule with the electronegative atom (F, O or N).
Cause of formation of Hydrogen Bond:
When hydrogen is bonded to strongly electronegative element X, the electrons pair shared between the two atoms moves far away from a hydrogen atom. As a result, the hydrogen atoms becomes highly electropositive with respect to the other atom ‘X’. Since there is displacement of electrons towards X, the hydrogen acquires fractional positive charge (δ+) while X attain fractional negative charge (δ–). This result in the formation of a polar molecule having electrostatic force of attraction which can be represented as:
![]()
This weak electrostatic attraction constitutes hydrogen bond. For example,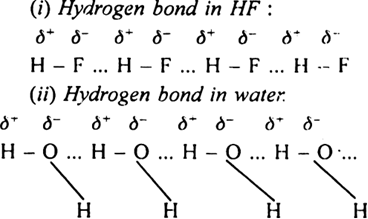
What are the conditions for hydrogen bonding ?
These are:
(i) Hydrogen atom should be bonded to highly electronegative atom (N, O, F). The strength of the hydrogen bond increases with an increase in the electronegativity of the other atom. For example, the electronegativity of N, O, F increases as N<OTherefore, the strength of hydrogen bond also increases as N – H ... N < O – H...O < F - H ... F.
(ii) The size of the electronegative atom should be small. Smaller the size of the atom, more the electrostatic force of attraction, stronger will be the hydrogen bond.
Water is a liquid while hydrogen sulphide is a gas. Explain.
Water exists as a liquid at room temperature with a high boiling point. Sulphur is less electronegative than oxygen, and the S-H bond is much less polar than the O-H bond. Hence, there is no hydrogen bonding in hydrogen sulphide, and it exists as a gas normally with discrete H2S molecules.
![]()
Out of NH3 and PH3, which has higher boiling point and why?
Nitrogen and chlorine have the same electronegativity but only former shows intermolecular hydrogen bonding. Discuss.
What are the types of hydrogen bonding? Give examples.
i) Intermolecular hydrogen bonding.
ii) Intramolecular hydrogen bonding.
(i) Intermolecular hydrogen bonding: It is defined as hydrogen bonding which occurs between different molecules of the same or different substances. As a result, the molecules join together to form a long chain. For example, hydrogen fluoride molecules join through intermolecular hydrogen bonding to form long zig-zag chains.of associated molecules.
(ii) Intramolecular hydrogen bonding: It is defined as hydrogen bonding which occurs between atoms contained in the same molecule. An intramolecular hydrogen bond results in the cyclisation of the molecules and prevents their association.
Few examples involving intramolecular hydrogen bonding are given below:

What are the conditions for the formation of intramolecular hydrogen bonding?
Intramolecular hydrogen bond: It is formed when a hydrogen atom is in between the two highly electronegative (F,O,N) atoms present within the same molecules. For example, in o- nitrophenol the hydrogen is in between the two oxygen atoms.
O-Nitrophenol is steam volatile, while p-nitrophenol is not. Explain.
Or
Boiling point of o-nitrophenol is less than p-nitrophenol. Explain.

Why does ice float over water?
We know that in ice, each oxygen atom is surrounded by four hydrogen atoms in such a way that the two hydrogen atoms are linked to an oxygen atom by covalent bonds whereas the other two hydrogen atoms are linked by hydrogen bonds. In ice (solid state), a water molecule is associated with four other water molecules through hydrogen bonding in a tetrahedral manner.
This gives rise to open cage-like structure which prevents the close packing of molecules (lower density). When ice absorbs heat and melts to form water, the hydrogen bonds break and close packing of water molecules takes place. Due to this close packing, the density of water is higher than that of ice and hence ice floats over water.
Give reasons for the following:
(i) Density of water is maximum at 277K (4°C).
(ii) Why glycerol (glycerine) is more viscous than ethyl alcohol?
(i) Ice has a cage-like structure with large empty space between the molecules. As water is warmed from 273K (melting point of ice) onward, more and more hydrogen bonds are broken. Naturally, water molecules come closer and acquire lesser volume. Hence, density increases. But this decrease in volume is opposed by the normal tendency for expansion on heating.
Up to 277 K: Contraction factor is more than the expansion effect, so there is a net decrease in volume or increase in density.
Above 277K : Expansion factor (due to increase in thermal energy) becomes greater than the contraction effect (caused by breaking of hydrogen bonds), naturally, volume increases and the density decreases. Thus, the density of water is maximum at 277 K.
(ii) This is because of the presence of three –OH groups in glycerol as compared to only one –OH group in ethyl alcohol. Hence, there are three hydrogen bonding sites in glycerol while there is only one in the case of ethyl alcohol molecule. Hence, there are greater intermolecular forces of attraction in glycerol as compared to ethyl alcohol, therefore, it is more viscous than ethyl alcohol.
Explain the formation of a chemical bond.
The attractive force which holds various constituents (atoms,ions, etc.)together in different chemical species is called a chemical bond.
Molecules having two identical atoms like H2 and O2 Cl2 N2 are called as homonuclear diatomic molecules.
Molecules containing two different atoms like CO, HCl, NO, HBr etc, are called as heteronuclear diatomic molecules.
Write Lewis symbols for the following atoms and ions:
S and S2–; Al and Al3+; H and H–.
Lewis symbol of following atoms and ions ,
S = 2, 8, 6
(Z = 16)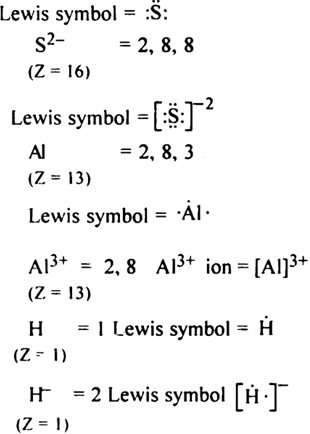
Define octet rule. Write its significance and limitations.
The tendency of atoms to complete eight electrons in their outer shell by interacting with other atoms through electron sharing or electrons transfer is known as the octet rule of chemical bonding.
Significance:
1. Octet rules able to explain chemical bonding in various compounds.
2. Octet rules able to illustrate the various type of bonds like a covalent, electrovalent and coordinate bond.
Limitation:
The octet rule is not satisfied for all atoms in a compound.
1. Compounds which have an incomplete octet of the central atoms.
LiCl, BeH2, BCl3, AlCl3,BF3, LiBr, AlBr3.
2.The rule failed to predict the shape and relative stability of molecules.
3. Octet rule fails to explain why in some compounds more than eight electron is there on central atom:
Compounds like PF5, SF6.
4. It is based upon the inert nature of noble gases. However, some noble gases like xenon and krypton form compounds such as XeF2, KrF2 etc.
Write three favourable factors for formation of ionic bond.
An Ionic bond is a type of chemical bond which involves the transfer of one or more electrons from one ion to another ion of opposite charges.
Three favourable factors for the formation of ionic bond are:
(i) Low ionisation enthalpy of the metal atom.
(ii) High electron gain enthalpy of the non- metal atom.
(iii) High lattice enthalpy of the compound formed.
Define bond length.

In the case of a covalent bond, the contribution from each atom is called covalent radius of that atom.
The bond length in a covalent molecule AB
R = r A + r B
where R is the bond length; rAand rB are the covalent radii of atoms A and B respectively.
Explain the important aspects of resonance with reference to the 
In some molecules, single Lewis structure fails to explain all the characteristics of the molecules. As a result, a number of structure can be drawn to explain all the characteristics. Thus, such structure is called resonating structure.
In  all C-O bonds are equivalent.This is possible only the different canonical structure of
all C-O bonds are equivalent.This is possible only the different canonical structure of  undergoing resonance to give resonance hybrid.
undergoing resonance to give resonance hybrid.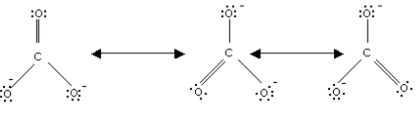
Define electronegativity. How does it differ from electron gain enthalpy?
Electronegativity is defined as the tendency of an element to attract the shared pair of electrons towards itself in a covalent. The is no specific units for electronegativity.It is only a relative tendency.
Electron gain enthalpy is the energy released when one mole of the electron are added to gaseous atoms of an element. It can be negative or positive depending upon whether the electrons is added or removed. An element has a constant value of the electron gain enthalpy that can be measured experimentally.
Explain with the help of suitable example polar covalent bond.
If two different atoms are linked to each other by a covalent bond,then the shared electron pair will not lie in the centre because the bonding atoms differ in electronegativities.Such a bond is called as polar covalent bond.For in NaCl , chlorine is more electronegative than sodium. Hence it will have more control over the shared pair of electrons & will develop a partial negative charge & sodium will acquire partial positive charge & a polar covalent bond is formed. Greater the difference in electronegativity of bonding atoms more will be the polarity of the bond.
What is meant by hybridization of atomic orbitals? Describe the shapes of sp, sp2, sp3 hybrid orbitals.
The phenomenon of mixing of orbitals of the same atom with a slight difference in energies so to redistribute their energies and give new orbitals of equivalent energy and shape. It is called as hybridization.
These hybrid orbitals have minimum repulsion between their electron pairs and thus, are more stable. Hybridization helps indicate the geometry of the molecule.
Sp3 hybridisation; This type of hybridization involves the mixing of one orbital of s- sub level or three orbitals of the p-sublevel of the valence shell to form four sp3 hybrid orbitals of equivalent energies and shape. Each sp3 hybrid orbital has 25% s character and 75% p-character. These hybridised orbitals tend to lie as apart in space as possible so that repulsive interaction between them are minimum. The four sp3 hybrid orbitals are directed towards the four corners of a tetrahedron. The angle between the sp3 hybrid orbitals is 109.5.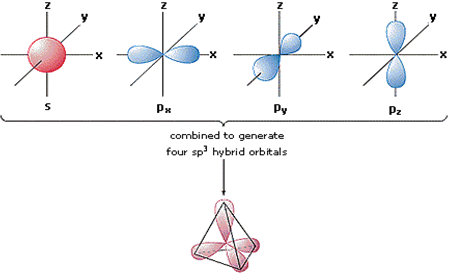
Sp2 hybridisation: This type of hybridization involves the mixing of one orbital of s- sublevel and two orbitals of p-sublevel of the valence shell to form three sp2 hybrid orbitals. These sp2 hybrid orbitals lie in a plane and are directed towards the corners of the equilateral triangle.
Each sp2 hybrid orbitals has one-third s- character and two -third p- character. sp2 hybridization is also called trigonal hybridisation.
sp-hybridisation: This type of hybridization involves the mixing of one orbital of the s-sublevel and one p-sublevel of the valence shell of the atom to form two sp- hybridised orbitals of equivalent shapes and energies. These sp hybridised orbitals are oriented in space at an angle of 180.
Distinguish between a sigma (σ) bond and a pi ![]() bond.
bond.
| Sigma (σ) bond | |
| 1. It is formed by the coaxial overlapping of two half filled atomic orbitals along the internuclear axis. | 1. It is formed by the sidewise or lateral overlapping of two half filled p-orbitals perpendicular to the intranuclear axis. |
| 2. This bond can be formed by the overlap of s –s.s –p and p-p orbitals. | 2. It involves the overlap of p-orbitals only i.e. s orbitals can not participate in the formation of |
| 3. Sigma bond is stronger and less reactive. | 3. |
| 4. They have cylindrical symmetry of electron density about the bond axis. | 4. Electron density is localised above and below the plane of the bond axis. |
| 5. Free rotation about a σ-bond is possible. | 5. Rotation of bond is restricted. |
| 6. Sigma bonds have an independent existence. | 6. |
Descibe the hybridisation in case of PCl5. Why are the axial bonds longer as compared to equatorial bonds?
Hybridization is the phenomenon of intermixing of atomic orbitals of slightly different energies of the atoms by redistributing their energies to form a new set of orbitals of equivalent energies and identical shape.
Atomic number of phosphorus P=15.
Electronic configuration of P
Ground state 1s2 2s2 3s2 3p3 3d0
Electronic configuration of P
excited state 1s2 2s2 3s1 3p3 3d1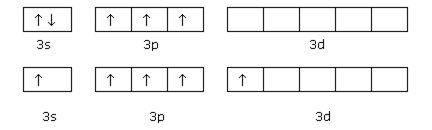
Now phosphorus has five empty shells, five Cl hybridised to form an equal set of equivalent five sp3d hybrid orbitals. These 5 sp3d orbitals are directed towards the five corners of a trigonal bipyramidal geometry.
In PCl5, out of the five hybrid orbitals, three orbitals form a P-Cl bond in one plane making and angle (P-Cl-P) 120 with each other. This plane is represented as equatorial plane and the bonds formed are equatorial bonds. Out of the remaining two hybrid orbitals, one lie perpendicularly above and the other lie perpendicularly above and the other lie perpendicularly below the equatorial plane, making an angle 90 with the plane and forms P-Cl bonds. These two bonds are called axial bonds. Since the axial bonds suffer more repulsive interaction from the equatorial bond pairs, they are found to be slightly longer and hence, slightly weaker than the equatorial bonds.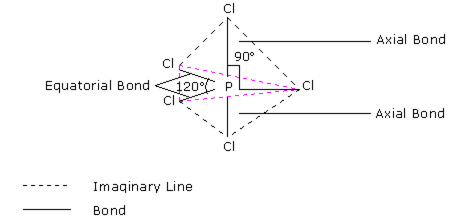
Define hydrogen bond. Is it weaker or stronger than the van der Waals forces?
Hydrogen bond represents a dipolar attraction between hydrogen atom & highly electronegative atom. The hydrogen bond is represented by ----------(dotted lines).
For eg H -----------X -----------X -------- X------
X = O,N,S,F,Cl….
The bond pair involved in hydrogen bonding was attracted more towards highly electronegative atom, as a result H atom will start acquiring a partial positive charge(δ+) & the electronegative atom will acquire a partial negative charge(δ-).
The magnitude of H-bonding is maximum in the solid state and minimum in the gaseous state.
Hydrogen bonds are of two types
(i) Intermolecular H-bond e.g., HF, H2O etc.
(ii) Intramolecular H-bond e.g., o-nitrophenol
Vander Waal's forces are weak forces and exist in non-polar compounds & noble gases .As compared to hydrogen bonds they are weak in nature because of strong dipole-dipole interaction in hydrogen bonding.
What is meant by the term bond order? Calculate the bond order of: ![]()
Bond order is defined as one-half of the difference between the number of electrons present in the bonding and anti-bonding orbitals of the molecule.
If Na is equal to the number of electron in an antibonding orbital, then
Nb is equal to the number of electron in a bonding orbital.
1/2(Nb-Na)
If Nb>Na, then the molecule is said to stable. However, if Nb
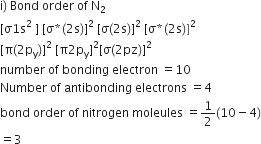

The ionic radii (in Å) of N3–, O2– and F– are respectively:
-
1.36, 1.40 and 1.71
-
1.36, 1.71 and 1.40
-
1.71, 1.40 and 1.36
-
1.71, 1.36 and 1.40
C.
1.71, 1.40 and 1.36
Number of electrons in N3- = 7+3 = 10
Number of electrons in O2- = 8+2 = 10
Number of electrons in F- = 9+1 = 10
Since, all the three species have each 10 electrons hence they are isoelectronic species.
It is considered that, in case of isoelectronic species as the negative charge increase, ionic radii increase and therefore the value of ionic radii are
N3- = 1.71 (highest among the three)
O2- = 1.40
F- = 1.36 (lowest among the three)
N3- > O2-> F-
In which of the following pairs of molecules/ions, both the species are not likely exist?
C.
species which have zero or negative bond order does not exist.
Which of the following exists as covalent crystals in the solid state?
-
Iodine
-
Silicon
-
Sulphur
-
Phosphorus
B.
Silicon
D.
Phosphorus
Silicon (Si) – covalent solid
Sulphur (S8) – molecular solid
Phosphorous (P4) – Molecular solid
Iodine (I2) – Molecular solid
Stability of the species Li2, Li2− and Li2+ increases in the order of:
-
Li2 < Li2+ < Li2-
-
Li2− < Li2+ < Li2
-
Li2< Li2− < Li2+
-
Li2− <Li2< Li2+
B.
Li2− < Li2+ < Li2
Li2+ is more stable than Li2− because Li2− has more numbers of antibonding electrons.
In which of the following pairs the two species are not isostructural?
-
CO32- and NO3-
-
PCl+4 and SiCl4
-
PF5 and BrF5
-
AlF63- and SF6
C.
PF5 and BrF5
i) CO32- and NO3- = Triangular Planar (sp2)
ii) PCl+4 and SiCl4 = Tetrahedral (sp3)
iii) PF5 and BrF5 = Tetrahedral (sp3)
iv) AlF63- and SF6 = sp3d2 hybridized, octahedral
Among the following, the maximum covalent character is shown by the compound
-
FeCl2
-
SnCl2
-
AlCl3
-
MgCl2
C.
AlCl3
The covalent character in ionic compounds is governed by Fazanís Rule.
(i) larger the charge on the ions.
(ii) smaller the size of anions.
(iii) larger the size of anion.
(iv) larger the polarizing power larger the covalent character.
AlCl3 will show Maximum covalent character on account of the higher polarising power of Al3+ because of its having higher positive charge and smaller size.
The hybridization of orbitals of N atom in NO3-, NO2+ and NH4+ are respectively
-
sp, sp2,sp3
-
sp2 , sp, sp
-
sp, sp3 , sp2
-
Sp2, sp3, sp
B.
sp2 , sp, sp
NO2+
Number of electron pairs = 2
Number of bond pairs = 2
Number of lone pair = 0
So, the species is linear with sp hybridisation.
NO3-
Number of electron pairs = 3
Number of bond pairs = 3
Number of lone pair = 0
So, the species is trigonal planar with sp2 hybridisation
NH4+
Number of electron pairs = 4
Number of bond pairs = 4
Number of lone pair = 0
So, the species is tetrahedral with sp3 hybridisation.
The group having isoelectronic species is
-
O2– , F–, Na+ , Mg2+
-
O– , F–, Na, Mg+
-
O2–, F–, Na ,Mg2+
-
O– , F–, Na+ , Mg2+
A.
O2– , F–, Na+ , Mg2+
| ions | O2- |
F– | Na+ | Mg+2 |
| Atomic number | 8 | 9 | 11 | 12 |
| No of electron | 10 | 10 | 10 | 10 |
The bond dissociation energy of B–F in BF3 is 646 kJ 1 mol− whereas that of C-F in CF4 is 515 kJ 1 mol−.The correct reason for higher B-F bond dissociation energy as compared to that of C-F is
-
Smaller size of B-atom as compared to that of C- atom
-
Stronger σ bond between B and F in BF3 as compared to that between C and F in CF4
-
Significant pπ - pπ interaction between B and F in BF whereas there is no possibility of such 3 interaction between C and F in CF4
-
lower degree of pπ - pπ interaction between B and F in BF3 than that between C and F in CF4
C.
Significant pπ - pπ interaction between B and F in BF whereas there is no possibility of such 3 interaction between C and F in CF4
B has vacant available p orbital and hence it involves pπ - pπ back bonding which is not possible in CF4 as C does not have vacant orbital. 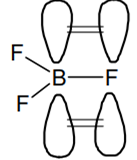
Which one of the following constitutes a group of the isoelectronic species?
B.
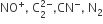
all have fourteen electrons.
Which one of the following pairs of species has the same bond order?
-
CN− and NO+
-
CN− and CN+
-
O2− and CN−
-
NO+ and CN-
A.
CN− and NO+
Both are isoelectronic and have same bond order.
Which of the following hydrogen bonds is the strongest?
-
O – H …… N
-
F – H …… F
-
O – H ….. O
-
O – H …… F
B.
F – H …… F
The hydrogen bond in HF is strongest, because fluorine is the most electronegative element.
In Fe(CO)5, the Fe – C bond possesses
-
π-character only
-
both σ and π characters
-
ionic character
-
σ-character only
B.
both σ and π characters
Which one of the following species is diamagnetic in nature?
-
He2+
-
H2
-
H2+
-
H2-
B.
H2
H2 σ1s2 σ* 1s0 , no unpaired so diamagnetic.
The number and type of bonds between two carbon atoms in calcium carbide are
-
One sigma, one pi
-
One sigma, two pi
-
Two sigma, one pi
-
Two sigma, two pi
B.
One sigma, two pi
σ =One π =Two
The structure of diborane (B2H6) contains
-
four 2c-2e bonds and two 3c-2e bonds
-
two 2c-2e bonds and four 3c-2e bonds
-
two 2c-2e bonds and two 3c-3e bonds
-
four 2c-2e bonds and four 3c-2e bonds
A.
four 2c-2e bonds and two 3c-2e bonds
Among Al2O3, SiO2, P2O3 and SO2 the correct order of acid strength is
-
SO2 < P2O3 < SiO2 < Al2O3
-
Al2O3 < SiO2 < P2O3 < SO2
-
Al2O3 < SiO2 < SO2 < P2O3
-
SiO2 < SO2 < Al2O3 < P2O3
B.
Al2O3 < SiO2 < P2O3 < SO2
While moving along a group from top to bottom acidic nature of oxides decreases and along a period left to right, acidic nature increases.
Al, Si, P, S
The bond order in NO is 2.5 while that in NO+ is 3. Which of the following statements is true for these two species?
-
Bond length in NO+ is greater than in NO
-
Bond length is unpredictable
-
Bond length in NO+ in equal to that in NO
-
Bond length in NO is greater than in NO+
D.
Bond length in NO is greater than in NO+
Bond length is inversely proportional to bond -order bond order in NO+ = 3, NO = 2.5
Thus bond length in NO> NO+
The states of hybridization of boron and oxygen atoms in boric acid (H3BO3) are respectively
-
sp2 and sp2
-
sp3 and sp3
-
sp3 and sp2
-
sp2 and sp3
D.
sp2 and sp3

Boron has three bonds thus sp2 hybridised. Each oxygen has two lone pairs hence sp3 hybridised.
The maximum number of 90° angles between bond pair of electrons is observed in
-
dsp3 hybridization
-
sp3d2 hybridization
-
dsp2 hybridization
-
sp3d hybridization
B.
sp3d2 hybridization
sp3d2 hybridisation has an octahedral structure such that four hybrid orbitals are at 90o w.r.t each other and others two at 90o with first four.
According to molecular orbital theory, which of the following will not be a viable molecule?
A.
| Species | Bond Order |
| 1/2[2-2] = 0 (does not exist) | |
| 1/2[2-0] = 1 (Exist) | |
| 1/2 [2-1] = 0.5 (Exist) | |
| 1/2 [ 2-1] = 0.5 (Exist) |
Which of the following compounds contain(s) no covalent bond(s)?
KCl, PH3, O2, B2H6, H2SO4
KCl, B2H6
KCl, B2H6, PH3
KCl, H2SO4
KCl
D.
KCl
KCl is an ionic compound.
PH3, O2, B2H6, H2SO4 covalent compound.
Total number of lone pair of electrons in I3- ion is
12
3
6
9
D.
9
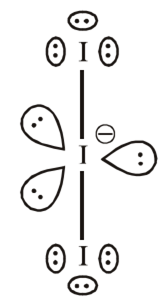
Total number of lone pair in I3- is 9.
Predict the correct order among the following.
-
long pair-lone pair> bond pair-bond pair> lone pair>bond pair
-
bond pair-bond pair> lone pair-bond pair > lone pair -lone pair
-
lone pair -bond pair > bond pair- bond pair> lone pair -lone pair
-
lone pair - lone pair > lone pair-bond pair > bond pair -bond pair
D.
lone pair - lone pair > lone pair-bond pair > bond pair -bond pair
According to the VSPER theory, a lone pair occupies more space than a bond pair, Hence when lone pair-lone pair interacts with each other they repel more and thus the correct order is:
lone pair - lone pair > lone pair-bond pair > bond pair -bond pair.
Which of the following species contains an equal number of σ and Π bond?
-
HCO3-
-
XeO4
-
(CN)2
-
CH2(CN)2
B.
XeO4
|
Structure |
σ and Π |
|
|
σ bond -4 |
|
|
σ bond -4 |
|
|
σ bond -3 |
|
|
σ bond -6 |
The total number of Pi bond electrons in the following structure is
-
4
-
8
-
12
-
16
B.
8
In a given structure there are 4 -pi bonds.Hence, total number of pi electrons are 8.
The pair of compounds that can exist together is
-
FeCl3.SnCl2
-
HgCl2,SnCl2
-
FeCl2,SnCl2
-
FeCl3,KI
C.
FeCl2,SnCl2
The compounds with lower oxidation number and which cannot reduce by one another can exist together. Thus, FeCl2 and SnCl2 can exist together as Fe2+ can not be reduced by Sn2+.
Which of the following molecules has the maximum dipole moment?
-
CO2
-
CH4
-
NH3
-
NF3
C.
NH3
CO2 and CH4 have zero dipole moment as these are symmetrical in nature. Between NH3 and NF3 , NF3 has greater dipole moment though in NH3 and NF3 both, N possesses one lone pair of electrons.
This is because, in the case of NH3, the net N-H bond dipole is in the same direction as the direction of the dipole of the lone pair but in the case of NF3, the direction of the net bond dipole of three -N-F bonds is opposite the that of the dipole of the lone pair.
Which one of the following species has the plane triangular shape?
-
N3
-
NO3-
-
NO2-
-
CO2
B.
NO3-
Species with sp2 hybridization are plane triangular in shape. Among the given species NO3- si sp2 hybridised with no lone pair of electrons on the central atom, N. whereas, N3, NO2- and CO2 are sp hybridised with a linear shape.
Magnetic moment 2.83 BM is given by which of the following ions?
(At. no.: Ti =22; Cr=24; Mn=25; Ni=28)
-
Ti3+
-
Ni2+
-
Cr3+
-
Mn2+
B.
Ni2+
Magnetic moment is given by
Hence,Ni2+ possesses a magnetic moment of 2.83 B.M
Which of the following organic compounds has same hybridization as its combustion product -(CO)2?
-
Ethane
-
Ethyne
-
Ethene
-
Ethanol
B.
Ethyne
Hybridisation of carbon = sp3
Which of the following species contains three bond pairs and one lone pair around the central atoms?
-
H2O
-
BF3
-
NH2-
-
PCl3
D.
PCl3

Thus, in PCl3 the central atom P atom three bond pairs and one lone pair.
The pair of species with t he same bond order is
-
O22-, B2
-
O2+, NO+
-
NO, CO
-
N2, O2
A.
O22-, B2

Which of the two ions from the list given below have the geometry that is explained by the same hybridization of orbitals,
D.

Hybridization of the given molecule is 
therefore,  both have the same hybridization.
both have the same hybridization.
The Correct order of increasing bond length of C - H, C-O, C - C and C = C is
-
C - C < C=C < C - O < C - H
-
C - O < C - H < C - C < C = C
-
C - H < C - O < C - C < C= C
-
C - H < C = C < C - O < C - C
D.
C - H < C = C < C - O < C - C
C - H: 0.109 nm
C = C : 0.134 nm
C - O: 0.143 nm
C - C : 0.154 nm
Therefore, Bond length order is
C - H < C = C < C- O < C - C
Which of the following is least likely to behave as Lewis base?
-
NH3
-
BF3
-
OH-
-
H2O
B.
BF3
BF3 is an electron deficient species, thus behaves like a Lewis acid.
Which of the following has the minimum bond length?
-
O2-
-
O22-
-
O2
-
O2+
D.
O2+

therefore, maximum bond order = minimum bond length.
Hence bond length is minimum for O2+
Decreasing order of stability of  is
is
A.

order of stability directly proportional to the bond order
therefore, the order of the stability of given species,
Which of the following structures is the most preferred and hence of lowest energy of SO3 ?
D.
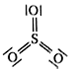
Formal charges help in the selection of the lowest energy structure from a number of possible energy structure from a number of possible Lewis structures for a given species. Generally, the lowest energy structure is the one with the smallest formal charges on the atoms.
Formal charge on an atom: = total number of valence electrons- non - bonding electrons - 1/2 x bonding electrons.
For lewis structure of SO3
Formal charge on three O atoms
In which of the following pairs, both the species are not isostructural?
-
SiCl4.PCl4+
-
Diamond, carbide
-
NH3, PH3
-
XeF4,XeO4
D.
XeF4,XeO4
a) SiCl4.PCl4+ : Both are isostructural because their central atom is sp3 hybridised and both have a tetrahedral arrangement.
b) Diamond and silicon carbide (SiC): Both are isostructural because their central atom is sp3 hybridised and both have a tetrahedral arrangement.
c) NH3 and PH3 have sp3 geometry.
d) XeF4 has sp3d2 hybridizations while XeO4 has sp3 hybridizations.Hence, XeF4 and XeO4 are not isostructural.
In which of the following pairs of molecules/ions, the central atoms have sp2 hybridization?
-
NO2- and NH3
-
BF3 and NO2-
-
NH2- and H2O
-
BF3 and NH2-
B.
BF3 and NO2-
(i) NO2- ⇒ 2σ + 1 lp =3, ie, sp2 hybridisation
(ii) NH3 ⇒ 3σ + 1 lp = 4, ie, sp3 hybridisation
(iii) BF3 ⇒ 3σ + 0 lp = 3, i.e sp2 hybridisation
(iv) NH2- ⇒ 2σ + 2 lp = 4 i.e sp3 hybridisation
(v) H2O ⇒ 2σ + 2 lp = 4 i.e sp3 hybridisation
Thus, among the given pairs, only BF3 and NO2- have sp2 hybridizations.
Which one of the following species does not exist under normal conditions?
-
Be2+
-
Be2
-
B2
-
Li2
B.
Be2
Molecules with zero bond order do not exist.
a) Be2+ (4 + 4 -1 = 7) = σ1s2 σ*1s2, σ2s2, σ2s1
BO = 4 - 3 / 2 = 0.5
BO = 4 - 4 / 2 = 0
c) B2 = (5 + 5) = 10
= σ1s2 , σ*1s2, 2σs2, σ*2s2, π2px1 = π2py1
BO = 6 - 4 /2 = 1
= σ1s2 , σ*1s2,σ2s2
BO = 4 - 2 / 2 = 1
Thus, bond order of Be2 does not exist under normal conditions.
In which one of the following species the central atom has the type of hybridisation which si not the same as that present in the other three?
-
SF4
-
I3-
-
SbCl52-
-
PCl5
C.
SbCl52-
Molecules having the same number of hybrid orbitals, have same hybridization and number of hybrid orbitals,
Since, only SbCl52- has a different number of hybrid orbitals (i.e,6) from the other given species, its hybridization is different front the others, ie, sp3d2.
The correct order of the decreasing ionic radii among the following isoelectronic species is
-
Ca2+ > K+ >S2- > Cl-
-
Cl- > S2- > Ca2+ > K+
-
S2- > Cl- > K+ > Ca2+
-
K+ > Ca2+ > Cl- > S2-
C.
S2- > Cl- > K+ > Ca2+

During the formation of a cation, the electrons are lost from the outer shell and the remaining electrons experience a great force of attraction by the nucleus, ie, attracted more towards the nucleus. In other words, nucleus holds the remaining electrons more tightly and result in decreased radii.
However, in the case of anion formation, the addition of an electron (s) takes place in the same outer shell, thus the hold of the nucleus on the electrons of outer shell decreases and this result in increased ionic radii.
Thus, the correct order of ionic radii is
S2- > Cl- > K+ > Ca2+
In which of the following molecules the central atom does not have sp3 hybridisation?
-
CH4
-
SF4
-
BF4-
-
NH4+
B.
SF4
When the number of hybrid orbitals, H is 4, the hybridization is sp3 hybridization
Thus, only in SF4 the central atom does not have sp3 hybridisation.
According to MO theory which of the following lists ranks the nitrogen species in terms of increasing bond order?
A.

Bond Order = 
Where
Nb = number of electrons in bonding MO
Na = number of electrons in antibonding MO
The state of hybridization of C2, C3, C5 and C6 of the hydrocarbon,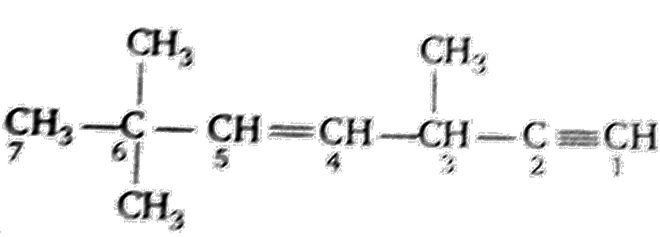
is in the following sequence
-
sp, sp3, sp2 and sp3
-
sp3, sp2, sp2 and sp
-
sp,sp2,sp2 and sp3
-
sp, sp2, sp3 and sp2
A.
sp, sp3, sp2 and sp3
Count a number of sigma bond (σ) and then find hybridization as follows.
If number of σ bonds = 2; hybridization is sp,
If number of σ bonds = 3; hybridization is sp2
if the number of σ bonds = 4; hybridization is sp3
Double and triple bond is not considered while finding hybridization.
In which of the following molecules/ions BF3, NO2-, NH2- and H2O, the central atom is sp2 hybridised?
-
NO2- and NH2-
-
NH2- and H2O
-
NO2- and H2O
-
BF3 and NO2-
D.
BF3 and NO2-
For sp2 hybridization, there must be either 3 sigma bonds or two sigma bonds and one lone pair of electrons in the molecules or ions.
In BF3 molecule, a number of sigma bond is 3 ie, sp2 hybridization.
In NO2- molecule, the number of sigma bond is 2 and the number of lone pairs is 2 ie, sp3 hybridization.
In NH2-molecule, the number of sigma bond is 2 and the number of lone pairs is 2 ie, sp3 hybridization.
In H2O molecule, the number of sigma bond is 2 and number of the lone pair are 2 ie, sp3 hybridization.
Thus, in BF3 and NO2- central atom is sp2 hybridised.
The angular shape of ozone molecule (O3) consists of
-
1 sigma and 2 pi bonds
-
2 sigma and 2 pi bonds
-
1 sigma and 1 pi bonds
-
2 sigma and 1 pi bonds
D.
2 sigma and 1 pi bonds
Single bond contains = 1 σ bond
Double bond contains = 1 σ bond + 1 π bond
Triple bond contains = 1σ bond + 2 π bond
The molecule of O3 is bent with an angle of 116.8o and equal O-O distance of 128 pm. The ozone molecule consists of 2 sigma bond and 1 pi bond.
The correct order of increasing bond angles in the following triatomic species is
B.

As the number of lone pair of electrons increases, bond angle decreases. NO2+ ion is isoelectronic with the CO2 molecule. It is a linear ion and its central atom (N+) undergoes sp-hybridisation, hence bond angle is 180o.
In NO2- ion, N -atom undergoes sp2 hybridisation. The angle between hybrid orbital should be 120o but one lone pair of electrons is lying on N- atom, hence bond angle decreases to 115o.
In NO2 molecule, N -atom has one unpaired electron in a sp2-hybrid orbital. The bond angle should be 120o but actually, it is 132o. It may be due to one unpaired electron in a sp2-hybrid orbital.
Therefore, the increasing order of bond angles.
In the hydrocarbon
The state of hybridization of carbon 1, 3, and 5 are in the following sequence
-
sp2, sp, sp3
-
sp, sp3, sp2
-
sp, sp2, sp3
-
sp3, sp2, sp
B.
sp, sp3, sp2

Hence the state of hybridization of carbon 1,3 and 5 are sp, sp3, sp2 respectively.
The correct order of C-O bond length among CO, CO32-, CO2 is,
-
CO2< CO32-< CO
-
CO < CO32-< CO2
-
CO32- <CO2 <CO
-
CO < CO2 < CO32-
B.
CO < CO32-< CO2
The bond length is the average distance between the centres of nuclei of two bonded atoms. Centres of nuclei of two bonded atoms. A multiple bonds (double or triple bond) is always shorter than the corresponding single bond.
The C- atom is CO32- is sp2 hybridised as shown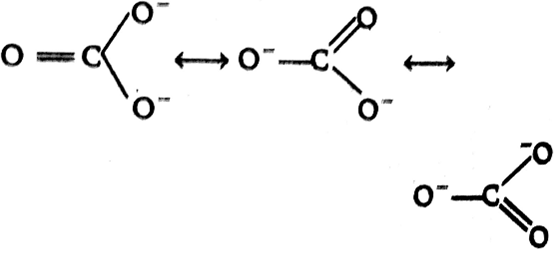
The C- atom is CO2 is sp hybridised with a bond distance of carbon -oxygen is 122 pm.
The C- atom in CO is sp hybridised with C-O bond distance is 110 pm:
So the correct order is
CO < CO32-< CO2
In which of the following pairs, the two species are isostructural?
-
SF4 and XeF4
-
SO32- and NO3-
-
BF3 and NF3
-
BrO3- and XeO3
D.
BrO3- and XeO3
a) SF4 = irregular tetrahedron (sp3d, one lone pair)
XeF4 = square planar (sp3d2, two lone pairs)
b) SO32- = pyramidal (sp3 one lone pair)
NO3- = trigonal planar (sp2)
c) BF3 = trigonal planar (sp2)
NF3 = pyramidal (sp3)
d) BrO3- = pyramidal (sp3, one lone pair)
XeO3 = pyramidal (sp3, one lone pair)
In which of the following molecules are all the bonds not equal?
-
ClF3
-
BF3
-
AlF3
-
NF3
A.
ClF3
In ClF3 all bonds are not equal due to trigonal -bipyramidal (sp3d-hybridisation) geometry of ClF3 molecule.
BF3 and AlF3 show trigonal symmetric structure due to sp2-hybridisation.
NF3 shows pyramidal geometry due to sp3 hybridization.
Which of the following is not isostructural with SiCl4?
-
SCl4
-
SO42- -
PO43-
-
NH4+
A.
SCl4
SCl4 is not isostructural of SiCl4 because it shows square planar structure due to the involvement of repulsion between lone pair and bond pair of electrons.
SO42- shows tetrahedral structural due to sp3 hybridization
PO43- shows tetrahedral structural due to sp3 hybridization
NH4+ shows tetrahedral structural due to sp3 hybridisation
With respect to the conformers of ethane, which of the following statements is true?
-
Bond angle remains same but bond length changes
-
Bond angle changes but bond length remains same
-
Both bond angle and bond length change
-
Both bond angles and bond length remains same
D.
Both bond angles and bond length remains same
There is no change in bond angles and bond lengths in the conformations of ethane. There is only change in dihedral angle.
Which of the following pairs of compounds is isoelectronic and isostructural?
-
BeCl2, XeF2
-
Tel2, XeF2
-
IBr2- , XeF
-
IF3, XeF2
C.
IBr2- , XeF
IBr2–, XeF2
The total number of valence electrons are equal in both the species and both the species are linear also.
Match the interhalogen compounds of column I with the geometry in column II and assign the correct code
| Column I | Column II |
| a) XX' | (i) T-shape |
|
b) XX3' |
(ii) Pentagonal |
|
c) XX'5 |
(iii) Linear |
| d) XX'7 | (iv) Square-pyramidal |
| (v) Tetrahedral |
-
(a) (b) (c) (d) (iii) (iv) (i) (ii) -
(a) (b) (c) (d) (iii) (i) (iv (ii) -
(a) (b) (c) (d) (iv) (v) (iii) (ii) -
(a) (b) (c) (d) (iv) (iii) (ii) (i)
B.
| (a) | (b) | (c) | (d) |
| (iii) | (i) | (iv | (ii) |
Which of the following is least soluble in water?
C2H6
CH3OH
CH3NH2
C6H5OH
A.
C2H6
C2H6 is non-polar compound and other compounds are polar in nature. Hence, it is least soluble in water.
Among the following compounds, which compound is polar as well as exhibit sp2-hybridisation by the central atom.
H2CO3
SiF4
BF4
HClO3
A.
H2CO3
SiF4 and HClO3 have sp3 - hybridisation.
BF3 and H2CO3 have sp2 - hybridisation but BF3 is non-polar.
Therefore, the Correct answer is H2CO3
Mock Test Series
Sponsor Area
Sponsor Area