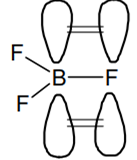
The bond dissociation energy of B–F in BF3 is 646 kJ 1 mol− whereas that of C-F in CF4 is 515 kJ 1 mol−.The correct reason for higher B-F bond dissociation energy as compared to that of C-F is
-
Smaller size of B-atom as compared to that of C- atom
-
Stronger σ bond between B and F in BF3 as compared to that between C and F in CF4
-
Significant pπ - pπ interaction between B and F in BF whereas there is no possibility of such 3 interaction between C and F in CF4
-
lower degree of pπ - pπ interaction between B and F in BF3 than that between C and F in CF4
C.
Significant pπ - pπ interaction between B and F in BF whereas there is no possibility of such 3 interaction between C and F in CF4
B has vacant available p orbital and hence it involves pπ - pπ back bonding which is not possible in CF4 as C does not have vacant orbital.