With the help of VSEPR theory, explain the shape of: (i) NH3 (ii) H2O.
 has five electrons in the valence shell. Three of these electrons are mutually shared with the electrons of three hydrogen atoms to form three N- H bonds as shown.
has five electrons in the valence shell. Three of these electrons are mutually shared with the electrons of three hydrogen atoms to form three N- H bonds as shown. 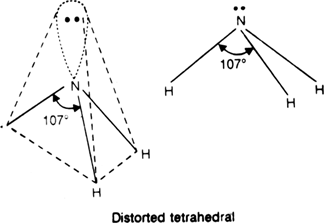
Hence, the central N atom in NH3 is surrounded by three bond pairs and one lone pair. The geometry expected for the molecule is tetrahedral since lone pair-bond pair repulsion is more than bond pair-bond pair repulsion. As a result, the lone pair of electrons will repel another pair strongly. Therefore three N–H bonds of NH3 are forced slightly closer.
This leads to decrease in H – N – H bond angles from a normal angle of a tetrahedron (109.5°) to 107°. The most favourable arrangement is distorted tetrahedral i.e. pyramidal. In this, nitrogen atom lies at the centre and three hydrogen atoms occupying the triangular base and the orbital with a lone pair of electrons from the apex of the pyramid.
(ii) H2O: In a water molecule, the central oxygen atom
 has six electrons in the valence shell. Two of these electrons are mutually shared with the electrons of two hydrogen atoms to form two O - H bonds.
has six electrons in the valence shell. Two of these electrons are mutually shared with the electrons of two hydrogen atoms to form two O - H bonds. Hence, the central oxygen atom is H2O is surrounded by two bond pairs and two lone pairs. These four electron pairs adopt tetrahedral arrangement. The presence of two lone pairs brings

distortion in the geometry of the molecule. The lone pairs repel the bond pairs more effectively resulting in the decrease of H – O – H angle from 109.5° to 104.5°. The water molecule may be regarded as bent or angular or V-shaped.



