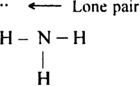Question
What do you understand by bond pairs and lone pairs of electrons? Illustrate by giving one example of each type.
Solution
The shared pairs of electrons present between the atoms are called bond pairs because they are responsible for bonding between the atoms. On the other hand, the valence electrons not involved in bonding are shown as such and are called lone pairs or unshared pairs or non-bonding electrons. For example, in ammonia molecule, there are four electron pairs, three of which are bond pairs and one is a lone pair.