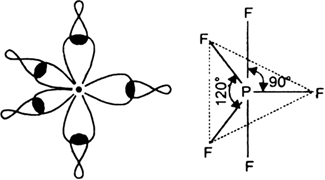
On the basis of hybridisation, explain the shape of phosphorus penta fluoride (PF5).
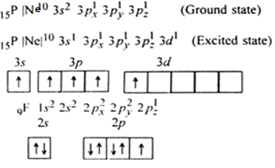
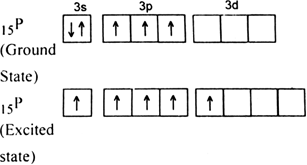
It is the case of sp3d hybridization in which one 3s, three 3p and one 3d orbital of P-atom having nearly same energy intermix to given five sp3 dihybrid orbitals. Now five hybrid orbitals are available for a combination which permits the formation of five covalent bonds with five fluorine atoms of PF5

But all the hybridised orbitals are not equivalent. Three of these are oriented towards the three corners of an equilateral triangle making an angle of 120° between them. The bonds formed by these orbitals are known as equatorial bonds. Remaining two orbitals are oriented at right angles to the plane of the first set of three orbitals. Thus PF5 has trigonal bipyramidal shape.