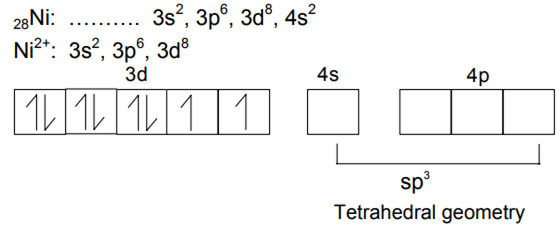
Nickel (Z = 28) combines with a uninegative monodentate ligand X–
to form a paramagnetic complex [NiX4 ]2 −. The number of unpaired electron(s) in the nickel and geometry of this complex ion are,
respectively
-
one, tetrahedral
-
two, tetrahedral
-
one, square planar
-
two, square planar
B.
two, tetrahedral