Draw the molecular orbital diagram for:
(i) Be2
(ii) B2 and predict bond order and magnetic properties.
(i) Be2 molecule: The electronic configuration of Be(Z = 4) is:
4 Be 1s2 2s1
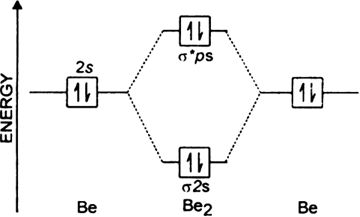
Be2 molecule is formed by the overlap of atomic orbitals of both beryllium atoms.
Number of valence electrons in Be atom = 2
Thus in the formation of Be2 molecule, two outer electrons of each Be atom i.e. 4 in all, have to be accommodated in various molecular orbitals in the increasing order of their energies.
.
The molecular orbital electronic configuration,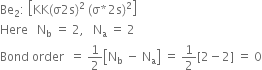
Magnetic property: Since bond order is zero, Be2 molecule does not exist. It is diamagnetic due to the absence of any unpaired electron.
B2 molecule: The electronic configuration of B atom (Z = 5) is![]()
B2 molecule is formed by the overlap of atomic orbitals of both boron atoms. A number of valence electrons of each boron atom = 3.
In the formation of B2 molecule, three valence electrons of each boron atom i.e. 6 in all, have to be accommodated in various molecular orbitals in the increasing order of their energies.
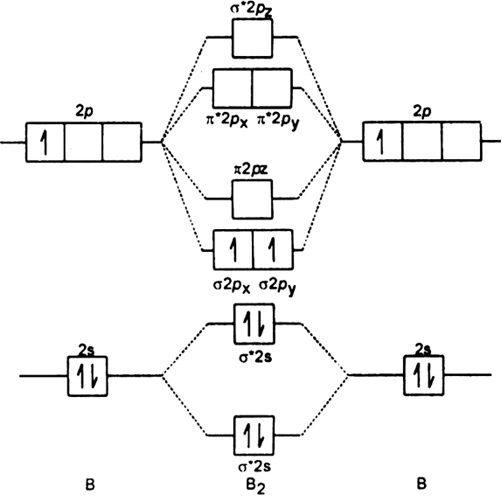
MO electronic configuration:![]()
Bond order: Here Nb = 4, Na = 2
Bond order = ![]()
The two boron atom is B2 molecules are linked by one covalent bond.
Magnetic properties: Since each ![]() 2px and
2px and ![]() 2py MO contains unpaired electron, therefore B2 molecule is paramagnetic.
2py MO contains unpaired electron, therefore B2 molecule is paramagnetic.



