Chemistry I Chapter 9 Coordination Compounds
Sponsor Area
NCERT Solution For Class 12 Business+studies Chemistry I
Write the formula for the following coordination compound:
Tetraaminediaquacobalt(lll)chloride
Write the formula for the following coordination compound:
Potassium tetracyanidonickelate(II)
Write the formula for the following coordination compound:
Tris(ethane -1, 2-diamine) chromium(III) chloride
Write the formula for the following coordination compound:
Amminebromidochloridonitrito-N-platinate (II)
Write the formula for the following coordination compound:
Dichloridobis(ethane-1, 2-diamine)platinum(lV) nitrate.
Write the formula for the following coordination compound:
Iron(III) hexacyanidoferrate(II)
Write the IUPAC names of the following coordination compounds:
(i) [Co(NH3)6]Cl3
(ii) [Co(NH3)5Cl]Cl2
(iii) K3[Fe(CN)6]
(iv) K3[Fe(C2O4)3]
(v) K2[PdCl4]
(vi) [Pt(NH3)2Cl(NH2CH3)]Cl.
(i) hexa-amine cobalt(III) chloride.
(ii) Penta amine chlorido cobalt(III) chloride.
(iii) Potassium hexacyano ferrate(III).
(iv) Potassium trioxalate ferrate(III).
(v) Potassium tetrachlorido palladate(II).
(vi) Diammine chlorido (methylamine) platinum (II) chloride.
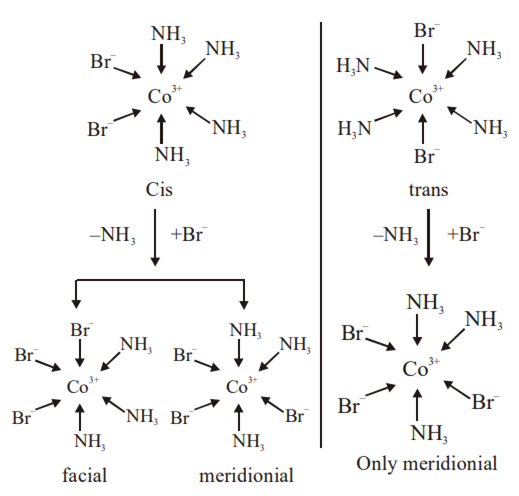
Indicate the type of isomerism exhibited by the following complexes and draw structures for these isomers:
(i) K[Cr(H2O)2(C2O4)2], (ii) [Co(en)3Cl3,
(iii) [Co(NH3)5(NO2)]|NO3]2, (iv) [Pt(NH3)(H2O)Cl2]
(i) Both geometrical (Cis, trans) and optical isomers for Cis can exist.
(ii) Two optical isomers can exist.
(iii) There are 10 possible isomers. There are geometrical, ionisation and linkage isomers possible.
(iv) Geometrical (Cis, trans) isomers can exist.
Give evidence that [Co(NH3)5Cl]SO4 and [Co(NH3)5SO4]Cl are ionisation isomers.
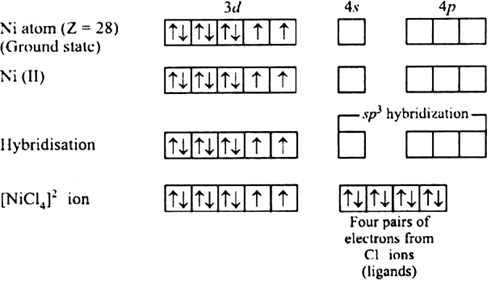
Explain on the basis of valence bond theory that [Ni(CN)4]2– ion with square planar is diamagnetic and the [NiCl4]2– ion with tetrahedral geometry is paramagnetic.
Ni is in the +2 oxidation state i.e., in d8 configuration.
There are 4 CN− ions. Thus, it can either have a tetrahedral geometry or square planar geometry. Since CN− ion is a strong field ligand, it causes the pairing of unpaired 3d electrons.
CN– will cause pairing of electrons. It is diamagnetic in nature due to the unpaired electron.
(ii) [Ni(Cl4)]2–
In case of [NiCl4] 2−, Cl− ion is a weak field ligand. Therefore, it does not lead to the pairing of unpaired 3d electrons. Therefore, it undergoes sp3 hybridization. Since there are 2 unpaired electrons in this case, it is paramagnetic in nature.
is paramagnetic while is diamagnetic though both are tetrahedral. Why?
is strongly paramagnetic whereas is weakly paramagnetic. Explain.
Explain is an inner orbital complex whereas is an outer orbital complex.
|
[Co(NH3)6]3+ |
[Ni(NH3)6]2+ |
|
Electronic configuration of cobalt = d6 |
Electronic configuration of Nickel = d8 |
|
In this compound oxidation state of cobalt is +3 |
In this compound oxidation state is +2 |
|
NH3 is a strong field ligand therefore it causes the pairing. Hence, Ni can undergo d2sp3 hybridization. Therefore, it is an inner orbital complex. |
If NH3 causes the pairing, then only one 3d orbital is empty. Thus, it cannot undergo d 2sp3 hybridization. Therefore, it undergoes sp3d 2 hybridization. Hence, it forms an outer orbital complex. |
Predict the number of unpaired electrons in the square planar [Pt(CN)4,]2– ion.

The hexaquo manganese(II) ion contains five unpaired electrons, while the hexacynoion contains only one unpaired electron. Explain using crystal field theory.
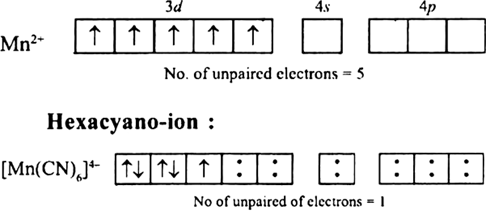
Hence, hexaaquo manganese (ll) ion has five unpaired electrons, while hexacyano ion has only one unpaired electron.
Calculate the overall complex dissociation equilibrium constant for the Cu(NH3)42+ion, given β4 for this complex is 2.1 x 1013.
β4 = 2.1 × 1013
The overall dissociation constant is the reciprocal of overall stability constant i.e.,
1/B4 = 1/2.1 x1013
= 4.7 x 10–14.
Identify the ligands from the following coordination compounds:
(a) [Co(en)2Cl(NO2)2]
(b) K[Co(CN)(CO2)(NO)]
(c) K3[Al(OH)6]
(d) [Co(H2O)2(NH3)4](OH)3
(a) Ethylene diamine, Cl– and NO2–.
(b) CN– , CO and NO.
(c) OH–
(d) H2O and NH3
Sponsor Area
Write IUPAC name of the following complexes:
(a) [Fe(H2O)6]SO4
(b) [Cu(en)2(NO3)2
(a) Hexa aqua iron sulphate(II).
(b) Bis-(ethane-l, 2-diamine) copper(II) nitrate.
Give IUPAC name of the following complexes:
(i) Na2[Fe(CN)5(NO)], (ii) K2[Zn(CN)4]
(i) Sodium pentacyano nitrosonium ferrate(II).
(ii) Potassium tetracyanozincate(II).
Provide systematic names to the following complexes:
(i) [Fe(H2O)5(NO)]SO4,
(ii) [Cr(NH3)6]3+
(iii) [Pt(NH3)2Cl2],
(iv) [NiCl4]2–
(i) Penta aquanitrosonium iron(I) sulphate.
(ii) Hexa amine chromium(III) ion.
(iii) Diamine dichloroplatinum(II).
(iv) Tetrachloronickelate(II) ion.
Write the chemical formula of:
(i) Sodium tetrahydridoborate(III).
(ii) Tetrahydroxo zincate(II) ion.
(ii) [ Zn(OH)4]2–.
What are the basic difference between the two geometrical isomers – cis/trans or fac/ mor?
Specify which out of the following complex structures exhibit geometrical isomerism:
(a) Linear, (b) Square planar, (c) Tetrahedral, (d) Octahedral.
Give two examples of positive mono-denate ligands.
NO+ (nitrosonium) and NH2NH3+ (hydrazinium).
Give an example of neutral bidentate ligand.
Ethylene diamine H2N-CH2-CH2-NH2 (represented as en). There are two coordination sites in it shown by lone pair of electrons at two nitrogen atoms.
Give an example of hexadentate ligand.

Why is geometrical isomerism not possible in tetrahedral complexes having two different types of unidentate ligands coordinated with the central metal ion ?
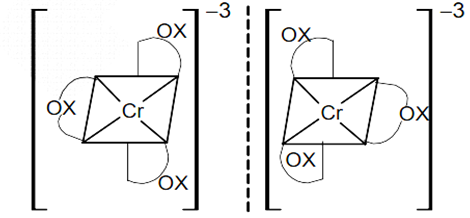
Write all isomers of [Co(C2O4)3]3–.
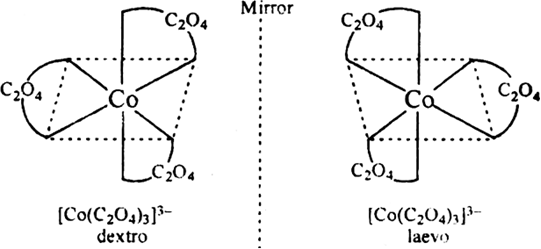
Fig. Isomers of [Co(C2O4)3]3–.
Draw structures of geometrical isomers of [Fe(NH3)2(CN)4]–.
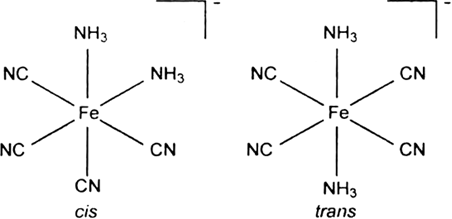
Fig. Geometrical isomers of [Fe(NH3)2(CN)4]–
Which isomer of [CoCl2(en)2]+ does not show optical isomerism?

Illustrate the geometrical isomers of [Pt(NH3)4Cl2]+.
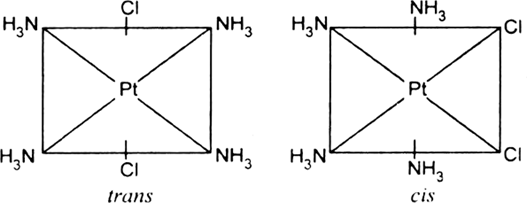
Fig. Geometrical isomers of [Pt(NH3)4Cl2]2+.
FeSO4 solution mixed with (NH4)2SO4 solution in 1 : 1 molar ratio gives the test of Fe2+ ion but CuSO4 solution mixed with aqueous ammonia in 1:4 molar ratio does not give the test of Cu2+ ion. Explain why?
FeSO4 +6H2O+(NH4)2SO4 --> FeSO4.(NH4)2SO4.H2O
But CuSO4 combines with NH3 to form the complex [Cu(NH3)4]SO4 in which the complex ion [Cu(NH3)4]2+does not dissociate to give Cu2+ions.
CuSO4 +4NH3 +5H2O-->[Cu(NH3)4]SO4.5H2O
How many coordination sites are there in ethylene diamine CH3NH2?
In ethylene diamine there is 2 coordination sites.
What are the most common coordination number encountered in coordination compounds?
The most common coordination number in coordination compound is 4 and 6.
What is the oxidation state of Ni in Ni(CO)4?
The oxidation state of Ni in Ni(CO)4 is Zero.
Give the geometry and magnetic character of [NiCl4]2–?
Magnetic character:- paramagnetic.
Give one example of hexadentate ligand. Give its use.

Sponsor Area
What is coordination number? Give coordination of Co in [Co(en)3]3+ ion.
Identify the ligands in [Co(en)2 Cl(ONO)]+ and write its IUPAC name.
Which of these cannot act as ligand and why: NH3, H2O, CO, CH4 ?
NH3 is strong ligand, NH4+ ion is not, why?
Write the IUPAC name for any of the isomers with the molecular formula [Pt(NH3)2Cl2]Cl2.
What is the coordination number of central metal ion in [Fe(C2O4)3]3–?
coordination of central metal ion is 6.
Name the type of isomerism exhibited by the following isomers.
[Pt(NH3)4] [PtCl6] and [Pt(NH3)4Cl2] [PtCl4]
Write the IUPAC name of [Co(en)2 (NH3)2]Cl3.
What are complex compounds? Give one example?
These are addition compound which retain their identity in the solid as well as in the solution forms. In these compound the individual properties of constituents are generally lost. A complex ferro- cyanide [Fe(CN)6]4- ion is formed when aqueous solution of Fe(CN)2 and KCN are mixed together. The salts of these complex ion are known as complex compounds or coordination compounds.
Write the formula of the following complexes:
(i) Hexa-amine platinum(IV) chloride.
(ii) Dichloro tetra amine cobalt(III) ion.
(ii) [CoCl2(NH3)4]+.
What is the coordination number and oxidation state of aluminium in the complex ion [Al(H2O)4(OH)2] +?
Oxidation state of aluminium is =3
Al=x
H2O=0
OH- =-1
Thus oxidation state is
x+0+(-2) =1
x= 2+1
x= 3.
Write all the isomers of |Pt(SCN) (NH3)3] (SCN).
The ligand SCN– is capable of linking itself through two atoms, viz S(–SCN) and N(–NCS), therefore the possible isomers are: [Pt(SCN)(NH3)3](SCN) and [Pt(NCS)(NH3)3] (SCN).
Why inner octahedral complexes are called low spin complexes?
Why outer octahedral complexes are celled high spin complexes?
As no pairing occurs in these complexes, they can have many unpaired electrons (from 1 to 5) and hence large value of magnetic moment. Therefore called high spin complexes.
Name the ligands which are regarded as strong crystal fields and causes pairing of electrons.
What is the crystal fields Theory?
What are the consequence of crystal fields?
How the coordinate entities show colour?
The colour of the coordination entity is decided on which factor?
The crystal field theory attributes the colour of the coordination compounds to d-d transition of the electron. The colour shown is always the complementary to the colour absorbed.
What is the oxidation state of gold in [Au(CN)2]–?
Oxidation of gold in [Au(CN)2]– is 1.
Au =x
CN =-1
Thus Oxidation state is be
x+(-2) = (-1)
x= 1
Which of the two is more stable K4[Fe(CN)6] or K3[Fe(CN)6].
where as in K3[Fe(CN)6] oxidation state is +3. Since both have strong field ligand therefore pairing occur. in case of +2 oxidation state all electron is paired such that the configuration is T2g6. But in case of +3 oxidation state the configuration is T2g5.Hence K3[Fe(CN)6] more stable because Fe3+ is smaller in size and has higher charge than Fe2+.
Write IUPAC name of K3[Fe(C2O4)3].
Give two complexes which are used in medicines.
(i) Cis-platin is used as anti-cancer agent.
(ii) Cyanocobalamine is vitamin B12 which is used to prevent anaemia.
[Fe(CN)6]3– is weakly paramagnetic while [Fe(CNO)6]4– is diamagnetic, why?
A coordination compound has the formula CoCl3.4NH3. It does not liberate NH3 but forms a precipitate of AgCl on treatment with AgNO3 solution. Write the structure and IUPAC name of the complex.
Explain why chelating complex is more stable than unchelated complex?
Write down the IUPAC names of the following compounds:
(i) [CO(NH3)5ONO ]Cl2
(ii) K3[Cr(CN)6]
(i) Penta amine nitrito cobalt(III) chloride.
(ii) Potassium hexa cyanochromate (III).
Give the name of a bidentate ligand.
When a ligand can bind through two donor atoms as in H2NCH2CH2NH2 (ethane-1,2-diamine) or
C2O42– (oxalate), the ligand is said to be bidentate ligand.
What is Wilkinson’s catalyst? Where is it used?
Nickel carbonyl( NiCO4) has tetrahedral geometry whereas [Pt(NH3)2Cl2] is square planar.
Write the expression for the stability’ constant for [Cu(NH3)4]2+.
.
Thus, Stability constant is
The value of equilibrium constants of [Cu(NH3)4]+ and [Co(NH3)6]3+ are 1.0 x 10–12 and 6.2 x 10–36 respectively. Which complex would be more stable and why ?
Solubility constant
Hence lower the value of equilibrium constant higher will be the stability, therefore, [Co(NH3)6]3+ will be more stable.
In octahedral complexes which two d-orbitals are used in hybridization.
Octahedral geometry arises due to d2sp3 or sp3d2 hybridisation of the central metal atom or ion. Octahedral complexes in which the central atom is d2sp3 hybridised are called inner- orbital octahedral complexes while the octahedral complexes in which the central atom is sp3d2 hybridised are called outer orbital octahedral.
If the value of Δ0 is less than P in the crystal field, write the arrangement of d4coordination entity in crystal field split.
How is ammonia molecule is a good ligand?
What do you understand by the term stability constant K for a complex?
Sponsor Area
There are two compounds [Cr(NH3)4]2+ and [Cr(CN)4]2– having 5.2 x 1011 and 3.2 x 1022 respectively as their K values, which complex is more stable and which ligand NH3or CN– is a stronger ligand?
What is the significance of Δ0?
The Δ0 relative values decides the actual configuration of the central metal atom/ion in the complex.
Write IUPAC name of the complex [Co(NH3)5SCN]Cl2.
Give the name of following complex using IUPAC norms:
[Co(en)2(ONO)Cl]Cl.
Give the IUPAC name of [NH4]3 [Co(ONO)6].
Write the IUPAC name of [Ni(H2O)6] (ClO4)2.
Name the ionisation isomer of [Cr(H2O)5Br]SO4.
Square planar complexes with coordination number of 4 exhibit geometrical isomerism whereas tetrahedral complexes do not. Why?
Why does [CoF6]3– give a high spin complex?
Cobalt exists in the +3 oxidation state.
Fluorine ion is a weak ligand. It cannot cause the pairing of the 3d electrons. As a result, the Co3+ ion will undergo sp3d2 hybridzation.
The complex formation involves d-orbitals of the outershell which give a high spin complex.[Cu(CN)4]2+ is more stable complex than [Cu(NH3)4]+. Why?
In aqueous solution Cu+ disproportionate to Cu2+ and Cu
2Cu+------>Cu2+ +Cu.
Square planar complexes do not show optical isomerism. Why?
The essential requirement for a compound to be optically active is that the compound should not have plane of symmetry in its structure. The tetra coordinated complexes with a square planar geometry contain a plane of symmetry. Therefore it do not show optical isomerism.
[Cu(NH3)4]2+ ion is coloured while [Cu(CN)]3– ion is colourless. Why?
[Cu(NH3)4]2+ has unpaired electron. Hence, [Cu(NH3)4]2+ ion is coloured whereas [Cu(CN)4]3–does not have unpaired electron. it is a colourless compound.
Identify the ligands in [Co(en)2 ClONO]+.
en = ethylene diamine
Cl= chlorine
ONO= Nitrito
What is the oxidation number of central atom in each of the following ?
(i) [Pt(NH3)6 Cl4 (ii) Fe4 [Fe(CN)6]3
(iii) [Ni(CO)4] (iv) Na[Hg(CN)3]
(i) [Pt(NH3)6 Cl4= +4
(ii) Fe4 [Fe(CN)6]3 =+2
(iii) [Ni(CO)4] = 0
(iv) Na[Hg(CN)3] = +1.
What is the coordination number of central metal atom in the following?
(i) [Pt(NH3)3]Cl (ii) K3[Fe(C2O4]3]
(iii) [Fe(EDTA)] (iv) [Rh{P(C6.H5)3}3]Cl
(i) [Pt(NH3)3]Cl = 6
(ii) K3[Fe(C2O4]3]= 6
(iii) [Fe(EDTA)] = 6
(iv) [Rh{P(C6.H5)3}3]Cl= 3
State the kind of isomerism possible for the following:
(i) [Cr(en)3]3+ (ii) [Cr(NH3)4 ClBr]Br (iii) [Cr(NH3)4 Br2l2SO4
(ii) [Cr(NH3)4 ClBr]Br = geometrical
(iii) [Cr(NH3)4 Br2l2SO4 =geometrical
Give names of two complexes which are used in medicines.
(i) EDTA is used in sol. the treatment of lead poisoning.
(ii) [Pt(NH3)2Cl2] known as cis-platin is used as an antitumor agnet in the treatment of cancer.
Write down the IUPAC names of the following compounds:
(i) [Co(NH3)5ONO]Cl2, (ii) K3[Cr(CN)6]
(i) Penta amine nitrito cobalt(lll) chloride.
(ii) Potassium hexa cyano chromate(III).
Through which atoms, C or O, the carbon monoxide ligand is attached to the central metal atoms in carbonyls?
Write the formula of the first f-block organometallic synthesised.
Where C5Me = Pentamethyl cyclopentadieny.
What is the oxidation state of the metal in the following coordiantion compound?
(i) Ni in Ni(CO)4 (ii) Co in [Co(CO) 4]–
(i) The oxidation state of nickel is zero.
Ni =x
CO=0
therefore oxidation state is 0
(ii) The oxidation state of cobalt is –1.
Co=x
CO=0
anion=+1
x+0=1
Therefore oxidation state is 1.
Which part of the d-block metals forms stable metal carbonyls?
If the geometry of [PtCl4]2– is square planar, which orbitals of Pt are involved in the bonding?


Hence, dsp2 hybridisation is taking place involving 5d, 6s and 6p.
What is the charge on the complex of Pt2+ in which the ligands are - one water molecule, one pyridine molecule and one ethylene diamine molecule?
The charge on complex ion is +2 because all ligands are neutal molecules.
Name the type of isomerism that occurs in complexes in which both cation and anion are complex ions.
Write down the IUPAC names of the following compounds:
(i) [Co(NH3)5ONO]Cl2, (ii) K3[Cr(CN)6]
(iii) [Cr(NH3)5CO3]Cl
The IUPAC names of the following compounds
(i) Penta amine nitrito cobalt(III) chloride.
(ii) Potassium hexa cyano chromate(III).
(iii) Penta amine carbonate chromium(lll) chloride.
Write formula of the following complexes:
(i) Penta amine chlorocobalt(II)
(ii) Lithium tetrahydro aluminate(III).
(i) [Co(NH3)5Cl]2+
(ii) Li[AlH4]
Which of the following will give white precipitate with AgNO3 solution:
[Pt(NH3)2Cl4], K[Pt(NH3)Cl5], [Pt(NH3)3 Cl3]Cl?
How many moles of ions are formed when one mole of the complex, [Co(NH3)4Cl2]Cl is completely dissociated into ions?
Only two ions are formed when this compound dissolves in water.
[Co(NH3)Cl2]Cl + H2O --> Co(NH3)4Cl2+(aq) + Cl-
Name a complex used in the treatment of cancer.
Write the name of any complex of copper in which the oxidation state of copper is one.
How many isomers are there for octahedral complex [CoCl2(en)(NH3)2]+
In the complex [SbF5]2–, sp3d hybridization is present. What would be the geometry of the complex ?
Give one example of each complex in which sulphate ion acts as mondenate and bidentate ligand.
[Co(en)2SO4]Br: In Bis-(ethylene diamine) sulphato cobalt(lll) bromide SO42– ion is acting as bidentate ligand.
What is the rule for writing the names of different ligands (neutral, negative etc.) during the naming of complexes?
What will be the correct for the wavelengths of absorption in the visible region for the following: , [Ni(NO2)6]4- , [Ni(NH3)6]2+, [Ni(H2O)6]2+?
The order of the ligand in the spectro-chemical series:
H2O < NH3 < NO2–
Hence, the wave lengths of the light observed will be in the order:
[Ni(H2O)6]2+ > [Ni(NH3)6]2+ < [Ni(NO2)6]4–
Thus, wavelength absorbed [E = hc/λ] will be in the opposite order.
Sponsor Area
Give one example each for inner orbital complex and outer orbital complex.
On the basis of the following observations made with aqueous solutions, assign primary and secondary valencies to metals in the following compounds:
|
Formula |
Motes of AgCl precipitated with excess AgNO3 |
|
(i) PdCl2.4NH3 |
2 |
|
(ii) NiCl2.6H2O |
2 |
|
(iii) PtCl4.2HCl |
0 |
|
(iv) CoCl3.4NH3 |
1 |
|
(v) PtCl2.2NH3 |
0 |
.
(i) Primary 2, secondary 4.
(ii) Primary 2, secondary 6.
(iii) Primary 4, secondary 6.
(iv) Primary 3, secondary 6.
(v) Primary 2, secondary 4.
Briefly write the rules for writing the formulas of mononuclear coordination compounds.
Mononuclear coordination compounds are the complex ions which have only one central metal atom or ion.
The formula of such complex compounds are written as per of the following rules:
(i) Central atoms is written first.
(ii) It is followed by the symbols of the ligands which are written in the order-negative ligands, neutral ligands followed by positive ligands.
(iii) If more than one type of ligands are there then their formulae are written according to the alphabetical order of the first symbol of their formulae.
(iv) The number of ligands linked to metal atom are indicated by putting its number as subscript written after simple brackets in which the lingands are placed.
(v) The coordination sphere is enclosed in square brackets.
(vi) No space is kept between representations of ions in the formula, whether the coordination sphere is a cation or anion.
(vii) If the formula of coordination sphere is written without the counter ion then the charge on the coordination sphere is written as a superscript on the right hand side, outside the square brackets, e.g., [Co(NH3)6]Cl3, [Cr(H2O)6]3+ etc.
Briefly explain the rules for writing down the names of the coordination compounds.
The rules which are to be followed while writing down the names of the coordination compounds are:
(i) The cationic part of the coordination compound is written first followed by the names of the anion with a small gap.
(ii) Within a coordination sphere, ligands names are written in alphabetical order before the name of the central metal atom or ion without leaving any gap.
(iii) To indicate the number of ligands in the coordination sphere two types of prefixes are used:
(a) One are di-, tri-, tetra- etc. These are used when the ligands names are simple.
(b) If the ligands are complex and contain the prefixes like di-, tri-, tetra etc. in their names, then prefixes -bis, -tris, tetrakis etc. are used.
(iv) The names of anionic ligands (both organic and inorganic) ends with –O–. If a ligand name is already having a prefix, its name is enclosed in simple brackets.
Neutral and cationic ligands names are written as such except – water (aqua), NH3 (–ammine), –CO (–carbonyl) and NO (nitrosyl).
(v) The oxidation state of the central atom or ion is indicated in Roman numerals after the name of the central atom in simple brackets with no gap in between.
(vi) When coordination sphere is an anion then -ate is added to the name of the central metal atom/ion.
What are ligands? Give examples.
What are ambident ligands? What are their importance?
Briefly classify the type of ligands on the basis of their ligating ability.
Ligand on the basis of their ligating ability can be classified as:
(i) Monodenate: When the ligands can donate the pair of electrons from one atom, it is called monodenate ligand, e.g., NH3, H2O, CN– etc.
(ii) Didentate: When the ligand can donate the pair of electrons through two atoms of the ligand, it is called didentate ligand, e.g., ethylene diamine![]()
(iii) Polydentate ligands : When the ligand has three or more atoms through which it can donate electron pairs, then the ligands are called polydentate ligands, e.g., edta(ethylene diamine tetra-acetate ion)![]()
What do you mean by coordination number? Explain with example.
The coordination number of a metal ion in a complex can be defined as the number of ligand donor atoms to which the metal is directly bonded.
The coordination number for a metal atom or ion is equal to the number of ligands attached to it. In case of monodendate ligands it attached by one donor atom and it is double in case of didentate ligands. Sometimes the sigma bond between the metal atom is represented by two dots before the donor atom, e.g., [Co(: (NH3)6)3+, [Ni(: CO)4].
What is a coordination polyhedron?
The spatial arrangement of the ligand atoms which are directly attached to the central atom/ion defines a coordination polyhedron about the central atom. The most common coordination polyhedra are octahedral, square planar and tetrahedral. For example, [Co(NH3)6]3+ is octahedral, [Ni(CO)4] is
tetrahedral and [PtCl4]2– is square planar.
What do you mean be denticity and chelation?
The number of atoms through which the ligands can donate a pair of electrons is called denticity of a ligand. Ligands on this basis can be unidentate, tredentate or polydentate.
When coordination of central metal atom or ion with ligand takes place from two or more groups of the same ligands in such a way that five or six membered rings are formed with metal atom or ion then it is called chelation and such ligands is called chelating ligands.
Therefore all didentate or polydentate ligands are chelating ligands.
Briefly explain the type of chelation.
Chelation can be of three major types:
(i) Didentate chelation: When chelation is done by didentate ligand it is called Didentate chelation, e.g., [PtCl2(en)] in this en is a didentate ligands.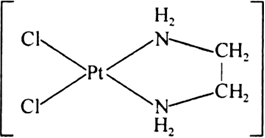
(ii) Terdentate chelation: When chelation is done by a terdentate (tridentate) ligand it is called tredentate chelation e.g., [PtCl(dien)+, dien is a terdentate ligand.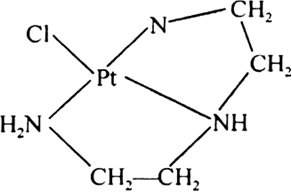
(iii) Tetradentate chelation: When chelation is done by tetradentate ligand it is called tetradentate chelation e.g., [Pt(trien)]2+, trien is a tetradentate ligand.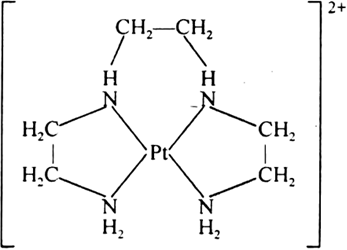
Write the formulas for the following coordination compounds:
(i) Tetraamine aqua chlorocobalt(III) chloride.
(ii) Potassium tetrahydroxozincate(II).
(iii) Potassium trioxalatoaluminate(III).
(iv) Dichloridobis (ethane-1, 2-diamine) cobalt(III) chloride.
(v) Tetracarbonyl nickel (0)
(i) [Co(NH3)4(H2O)Cl]Cl2
(ii) K2[Zn(OH)4]
(iii) K3[Al(C2O4)3]
(iv) [CoCl2(en)2]Cl
(v) [Ni(CO)4]
Write the IUPAC names of the following coordination compounds:
(i) [Pt(NH3)2Cl(NO2)]
(ii) K3[Cr(C2O4)3]
(iii) [CoCl2(en)2]Cl
(iv) [Co(NH3)5(CO3)]Cl
(v) Hg[Co(SCN)4]
(i) Diamine chloronitrito-N-platinum(II).
(ii) Potassium trioxalato chromate(III).
(iii) Dichloro bis (ethane–1, 2–diamine) cobalt(III) chloride.
(iv) Hexa amine carbonato cobalt(III) chloride.
(v) Mercury tetrathiocyanato cobaltate(III).
A solution of [Ni (H2O)6]2+ is green but a solution of [Ni(CN)4]2– is colourless. Explain.
[Fe(CN)6]4- and [Fe(H2O)6]2+ are of different colours in dilute solutions. Why?
What will be the correct order for the wavelength of absorption in the visible region for the following:
[Ni (NO2)6]4–, [Ni(NH3)6]2+, [Ni(H2O)6]2+?
The central metal atom is same in all compound. Thus adsorption in the visible reigon depend on the ligand. according to spectrochemical series increasing order of ligand is H2O < NH3 < NO2-. Hence the correct order for wavelength is
[Ni (NO2)6]4–<[Ni(NH3)6]2+<[Ni(H2O)6]2+.
(i) [Ni– (NO2)6]4– = wavelength of light is 498 nm.
(ii) [Ni(NH3)6]2+= wavelength of light is 475 nm.
(iii) [Ni(H2O)6]2+ = wavelength of light is 500nm.
Out of the following two coordination entities which is chiral (optically active)?
(a) cis-[CrCl2(ox)2]3+
(b) [trans-[CrCl2 (ox)2]3–

Fig. Coordination entities.
Out of the two (a) cis-[CrCl2(OX)2]3– is chiral (optically active).
Explain the bonding in coordination compounds in terms of Werner’s postulates.
Postulates are:
(i) In coordination compounds metals show two types of linkages (valencies) - primary and secondary.
(ii) The primary valencies are normally ionisable and are satisfied by negative ions.
(iii) The secondary valencies are non-ionisable. These are satisfied by neutral molecules or negative ions. The secondary valence is equal to the coordination number and is fixed for a neutral.
(iv)The ions/groups bound by the secondary linkages to the metal have characteristic spatial arrangements corresponding to different numbers.
In the modern formulations, such spatial arrangements are called coordination polyhedra. The species with the square brackets are coordination entities or complexes and the ions outside the square bracket are called counter ions.
He further postulated that octahedral, tetrahedral and square planar geometrical shapes are more common in coordination compounds of transition metals. Thus [Co(NH3)6]3+, [CoCl(NH3)5]2+ and [CoCl2(NH3)4]+ are octahedral entities, while [Ni(Co)4] and [PtCl4]2– are tetrahedral and square planar respectively.
Explain with two examples each of the following: coordination entity, ligand, coordination number, coordination polyhedron, homoleptic and heteroleptic.
Coordination entity : A coordination entity constitutes a central atom/ion, usually of a metal, to which are attached a fixed number of other atoms or groups each of which is called a ligand. It may be neutral or charged.
Examples: [Co(NH3)6]3+, [PtCl4]2–, [Fe(CN)6]3–, [NiCl2(OH2)4]
Ligand: The groups attached to the central metal ion (or atom) in a complex are called ligands. The ligands may be anions like CN–, C–, C2O42– ion neutral molecules like H2O, NH3, CO. Irrespective of their nature all types of ligands have lone pair of electrons.
Coordination number: Total number of ligand atoms which are bound to a given metal ion is called its coordination number. Coordination number of a metal ion is also equal to the total number of coordinate bonds present in a complex.
Coordinations polyhedron: The spatial arrangement of the ligand atoms which are directly attached to the central atom/ion defines a coordination polyhedron about the central atom. The most common coordination polyhedra are octahedral, square planar and tetrahedral. For example, [Co(NH3)6]3+ is octahedral, [Ni(Co)4] is tetrahedral and [PtCl4]2– is square planar.
Homoleptic: Complexes in which a metal is bound to only one kind of donor groups, e.g., [Co(NH3)6]3+, are known as homoleptic.
Heteroleptic: Complexes in which a metal is bound to more than one kind of donor groups, e.g., [Co(NH3)4Cl2]+, are known as heteroleptic.
What is meant by unidentate, didentate and ambidendate ligands ? Give two examples of each.
Unidentate: When the ligands can donate the pair of electrons from one atom, it is called unidentate ligands, e.g., NH3, H2O, CN– etc.
Didentate : When the ligand can donate the pair of electrons through two atoms of the ligand, it is called didentate ligand.
Ambidendate: It is that unidentate ligand which can ligate through two different atoms present in it to central atom/ion giving two different coordination entity. Examples are NO2– and SCN– ions. NO2– can ligate through either N or O atom and SCN can ligate through S or N atom to central atom/ion of coordination entity. This results into formation of linkage isomers.
For example : NO2– group can do coordination to metal ion through or atom forming nitro complex or through oxygen atom forming nitrito complex.
[Co(NH3)5(–NO2)]2+ and [Co(NH3)5(–ONO)]2+.
Specify the oxidation numbers of the metals in the following coordination entities:
[Co(H2O)(CN)(en)2]2+
Calculation of oxidation number of the metal in the coordination entities:
Co =x
H2O =0
CN= (-1)
en =0
thus oxidation number is:
x + 0 + (– 1) + 0 = +2
∴ x = 3
oxidation no. of Co = 3
Specify the oxidation numbers of the metals in the following coordination entities:
[PtCl4]2–
The oxidation number of the metals in coordination entities:
Pt= x
Cl=(-1)
Thus oxidation number is:
x + 4 (–1) = –2
or x = 2
oxidation no. of Pt = 2
Specify the oxidation numbers of the metals in the following coordination entities:
[CoBr2(en)2]+
The oxidation numbers of the metals in coordination entities:
Co = x
Br=(-1)
en=0
thus oxidation number is
x + 2(–1) + 0 = +1
or x = 3
oxidation no. of Co = 3
Specify the oxidation numbers of the metals in the following coordination entities:
K3[Fe(CN)6]
The oxiation number of the metals in the coordination entities:
Fe =x
CN=(-1)
K=1
Thus oxidation number is
3(+1)+ x + 6(–1) = 0
or x = 3
oxidation no.of Fe = 3
Specify the oxidation numbers of the metals in the following coordination entities:
[Cr(NH3)3Cl3]
The oxidation number of the metal in the coordination entities:
Cr =x
NH3 =
Cl =(-1)
Thus the oxidation number is
x + 0 + 3(–1) = 0
or x = 3
oxidation no. of Cr = 3
Using IUPAC norms write the formulas of the following:
Diamminedichloridoplatinum(II).
Using IUPAC norms write the formulas of the following:
Pentaamminenitrito–O–cobalt(III).
Using IUPAC norms write the formulas of the following:
Hexaamminecobalt(III) sulphate
Using IUPAC norms write the formulas of the following:
Potassium tri(oxalato)chromate(III)
Using IUPAC norms write the formulas of the following:
Pentaamminenitrito–N–cobalt(III)
Using IUPAC norms write the systematic name of the following:
[Co(NH3)6]Cl3
Using IUPAC norms write the systematic name of the following:
[Pt(NH3)2Cl(NH2CH3)]Cl
Using IUPAC norms write the systematic name of the following:
[Ti(H2O)6]3+
Using IUPAC norms write the systematic name of the following:
[Co(NH3)4Cl(NO2)]Cl
Using IUPAC norms write the systematic name of the following:
[Mn(H2O)6]2+
Using IUPAC norms write the systematic name of the following:
[NiCl4]2-
Tetrachloridonickelate(II) ion
Using IUPAC norms write the systematic name of the following:
[Ni(NH3)6]Cl2
Using IUPAC norms write the systematic name of the following:
[Co(en)3]3+
Using IUPAC norms write the systematic name of the following:
[Ni(CO)4]
List various types of isomerism possible for coordination compounds, giving an example of each.
are ionisation isomers.
(ii) Coordination isomerism: This type of isomerism occurs when both the cation and anion are complexes and they differ in the coordination of ligands, e.g., [Co(NH3)6][Cr(C2O4)3] and [Cr(NH3)6] [Co(C2O4)3] are coordination isomers.
(iii) Linkage isomerism: The isomerism in which a ligand can form linkage with metal through different atoms, e.g., nitro group can link to metal either through nitrogen (–NO2) or through oxygen atom, e.g.,
and
are linkage isomers.
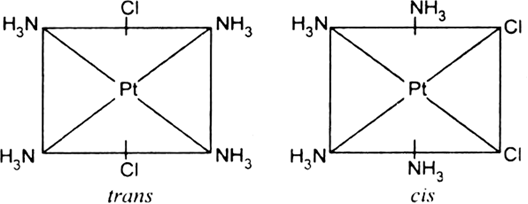
(iv) Geometrical isomerism: In tetra coordinated square planar complexes, cis- (when same groups are on same side and trans- (when same groups are on opposite sides) isomers are possible depending on position of different ligands, e.g., cis-platin and trans-diamine dichloro platinum(II).
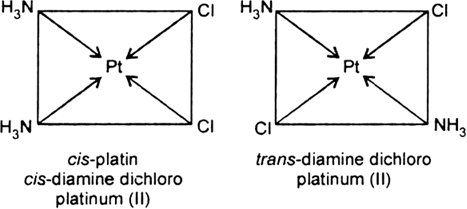
Fig. Geometrical isomerism.
(v) Optical isomerism: Optical isomers are those which are not superimposable on their mirror images. Complexes with coordination number six, having bidentate ligands provide examples of optical isomerism, e.g.,
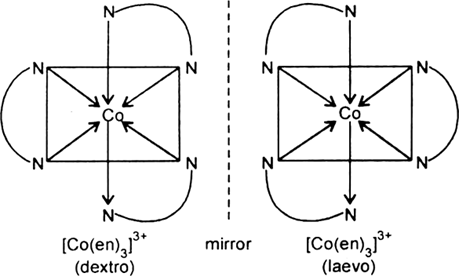
Fig. Optical isomerism.
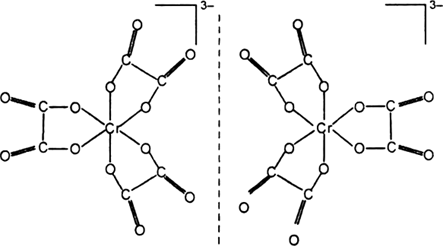
How many geometrical isomers are possible in the following coordination entities?
[Cr(C2O4)3]3–
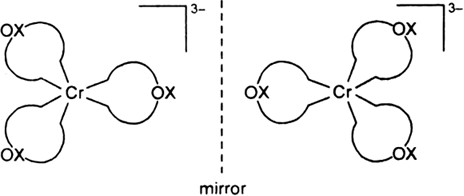
Fig. Geometrical isomers [Cr(C2O4)3]3– .
How many geometrical isomers are possible in the following coordination entities?
[Co(NH3)3Cl3].
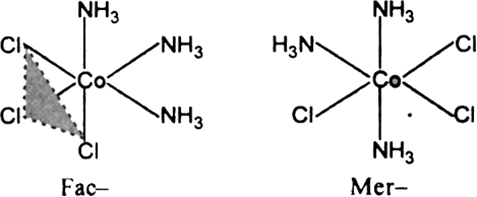
Fig. Geometrical isomers of [Co(NH3)3Cl3].
Two geometrical isomers Facial and Meridional are possible.
Draw the structures of optical isomers of:
[Cr(C2O4)3]3–
dextro laveo
Fig. Optical isomers of [Cr(C2O4)3]3–
Draw the structures of optical isomers of:
[PtCl2(en)2]2+
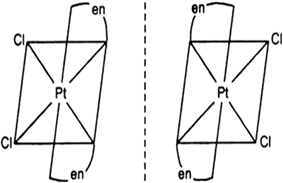
fig: optical isomers of [PtCl2(en)2]2+
Draw the structures of optical isomers of:
[Cr(NH3)2Cl2(en)] +
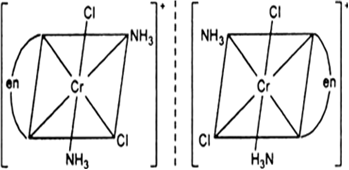
Fig: optical isomers of [Cr(NH3)2Cl2(en)]+
Draw all the isomers (geometrical and optical) of
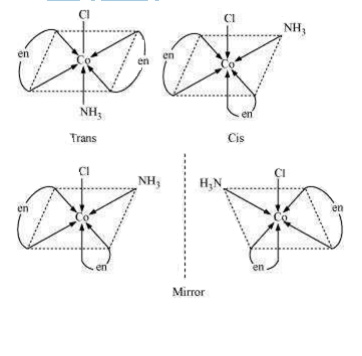
[CoCl2(en)2] +
[CoCl2(en)2]+
Two geometrical isomers cis- and trans- forms are possible.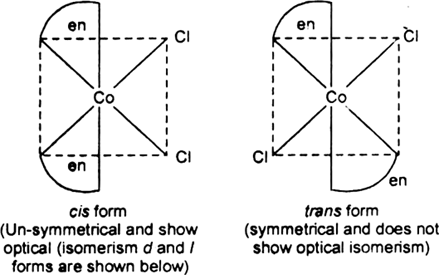
Optical isomerism dextro and levo shown by cis form: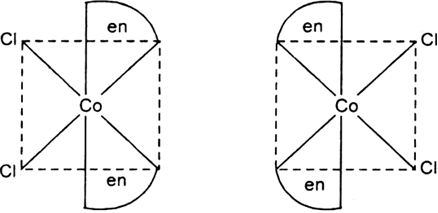
Fig. dextro and levo forms of cis [CoCl2(en)2]+
Draw all the isomers (geometrical and optical) of
[Co(NH3)Cl(en)2 ]2+
[CoCl(en)2(NH3)]2+
Two geometrical isomers, cis and trans forms are possible
Draw all the isomers (geometrical and optical) of
[Co(NH3)2Cl2(en)]+
isomers of [Co(NH3)2Cl2(en)]+
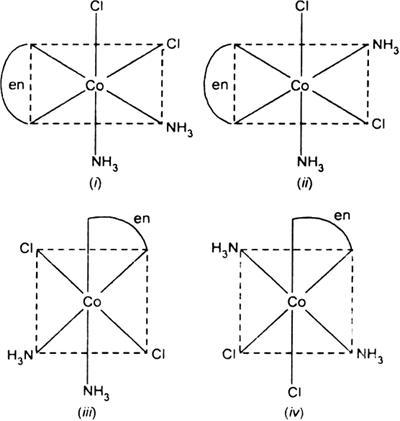
Write all the geometrical isomers of[Pt(NH3)(Br)Cl(py)] and how many of these will exhibit optical isomers?

Optical isomerism:

Aqueous copper sulphate solution (blue in colour) gives:
(i) a green precipitate with aqueous potassium fluoride and
(ii) a bright green solution with aqueous potassium chloride. Explain these experimental results.
Aqueous copper sulphate contains coordination entities [Cu(H2O)4]2+ which are blue in colour. Water molecule is a weaker ligand than Cl– and F–.
(a) On addition of aqueous KF solution, a new complex entity is formed which is of green colour.
(b) On addition of aqueous solution of KCl, an another complex entity is formed which is soluble in water.
What is the coordination entity formed when excess of aqueous KCN is added to an aqueous solution of copper sulphate? Why is it that no precipitate of copper sulphide is obtained when H2S(g) is passed through this solution?
CuSO4 (aq) + 4KCN → K2[Cu(CN)4] + K2SO4(aq)
Cyanide ligand CN– is a strong field ligand and stability constant of [Cu(CN)4]2– is quite large and thus practically no Cu2+ ions are left in solution. Hence, no precipitate of copper sulphide is obtained when H2S(g) is passed through solution.
Draw figure to show splitting of d orbitals in an octahedral crystal field.
Crystal field effects in octahedral coordination entities:
(i) Let us assume that the six ligands are positioned symmetrically along the cartesian axes, with metal atom at the origin. As the ligands approach first there is an increase in energy of d-orbitals relative to that of the free ion just as would be the case in a spherical field.
(ii) The orbitals lying along the axes (dz2 , and dx2– y2) get repelled more strongly than d xy’, d yz. and d zx orbitals which have lobes directed between the axes.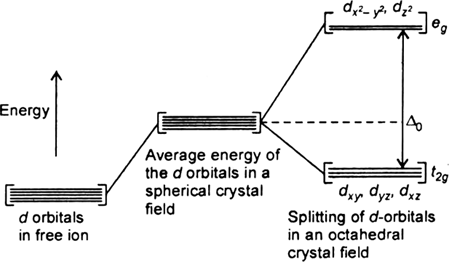
Fig. d-orbital splitting in an octahedral crystal field.
The dz2 , and dx2– y2 orbitals get raised in energy and dxy, dyz, dxz orbitals are lowered in energy relative to the average energy in the spherical crystal field.
Thus, the degenerate set of d-orbitals get split into two sets: the lower energy orbitals set t2g and the higher field energy orbitals eg set. The energy is separated by Δ0.
What is spectrochemical series? Explain the difference between a weak field ligand and a strong field ligand.
Spectro-chemical series is a series in which the ligands have been arranged in order of increasing magnitude of splitting they produce. The order is
I– < Br– < SCN– < Cl– < S2– < F – < OH– < C2O42– < H2O < NCS–< edta4– < NH3 < en < CN– < CO
The ligand present on the R.H.S of the series are strong field ligand while L.H.S are weak field ligand. Also, strong field ligand cause higher splitting in the d- orbitals than weak field ligand .
Weak field ligand | Strong field ligand |
1.They are formed when the crystal field stabilisation energy (Δ0) in octahedral complexes is less than the energy required for an electron pairing in a single orbital (p).
| 1. They are formed when the crystal field stabilisation energy (Δ0) is greater than the p.
|
2. They are also called high spin complexes.
| 2. They are called low spin complexes.
|
3. They are mostly paramagnetic in nature complex.
| 3. They are mostly diamagnetic or less paramagnetic than weak field.
|
Discuss the nature of bonding in the following coordination entities on the basis of valence bond theory.
[Fe(CN)6]4–
[Fe(CN)6]4 –
Outer electronic configuration of iron (Z = 26) in ground state is 3d64s2. Iron in this complex is in +2 oxidation state. Iron achieves +2 oxidation state by the loss of two 4s electrons. The resulting Fe2+ ion has outer electronic configuration of 3d6.
Since CN- is strong field ligand thus cause pairing.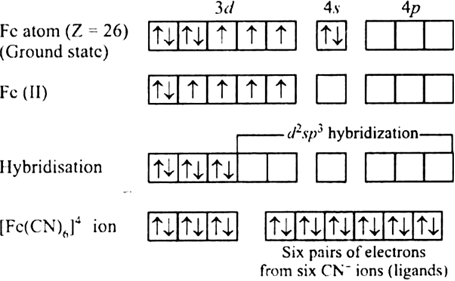
It has been experimentally observed that this complex is diamagnetic as such has no unpaired electron. To account for this two unpaired electrons in the 3d - subshell pair up, thus leaving two 3d-orbitals empty. These two vacant 3d-orbitals along with one 4s-orbital and three 4p-orbitals hybridise to give six equivalent d2sp3 hybridised orbitals. Six pairs of electrons, one from each cyanide ion, occupy the six vacant hybrid orbitals so produced. The resulting complex ion has an octahedral geometry and is diamagnetic due to the absence of unpaired electrons.
Discuss the nature of bonding in the following coordination entities on the basis of valence bond theory.
[FeF6]3–
Bonding in [FeF6]3–
The oxidation state of Fe is +3 and its coordination number is 6. The complex has octahedral geometry and experimental study shows that it is paramagnetic.
The bonding in this entity is explained on the basis of overlap of sp3d2 hybrid orbitals of Fe3+ ion and six lone pair orbitals of cyanide ligands. Five 3d electrons are unpaired which make it paramagnetic. The outer electronic configuration of Fe3+ ion is 3d5 which is highly stable and no pairing of electrons takes place in presence of weak field of F– ions. Here, 4d–orbitals of Fe3+ (which are empty) are involved.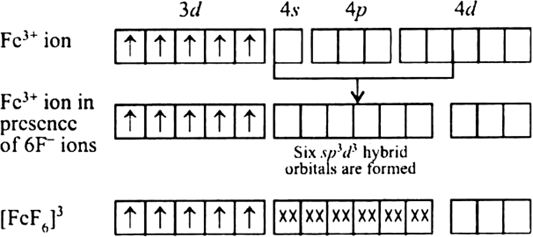
The entity is strongly paramagnetic due to five unpaired electrons and is an outer orbital complex.
Discuss the nature of bonding in the following coordination entities on the basis of valence bond theory.
[CoF6]3–
Fluorine ion is a weak ligand. It cannot cause the pairing of the 3d electrons. As a result, the Co3+ ion will undergo sp3d2 hybridzation.
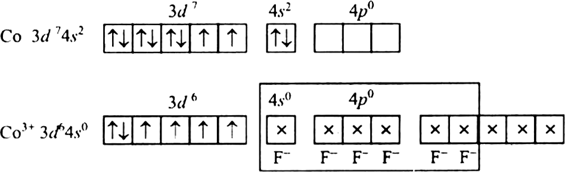
Hence, the geometry of the complex is octahedral and paramagnetic.
What is crystal field splitting energy? How does the magnitude of Δ0 decide the actual configuration of d orbitals in a coordination entity?
The degenerate d-orbitals (in a spherical field environment) split into two levels i.e., eg and t2g in the presence of ligands. The splitting of the degenerate levels due to the presence of ligands is called the crystal-field splitting while the energy difference between the two levels (eg and t2g) is called the crystal-field splitting energy. It is denoted by ∆o.
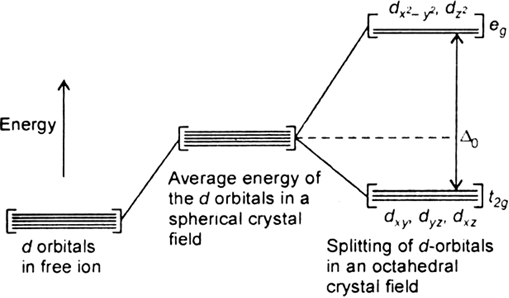
Fig. d-orbital splitting in an octahedral crystal field.
The formation of complex depend on the crystal field splitting, ∆o and pairing energy (P).
i)If ∆o < P, the fourth electron enters one of the eg orbitals giving theconfiguration t2g3. Ligands for which ∆o < P are known as weak field ligands and form high spin complexes.
ii) If ∆o > P, it becomes more energetically favourable for the fourth electron to occupy a t2g orbital with configuration t2g4 eg0. Ligands which produce this effect are known as strong field ligands and form low spin complexes.
[Cr(NH3)6)3+ is paramagnetic while [Ni(CN)4]2 – is diamagnetic. Explain why?

[Ni(CN)4]2– : Outer electronic configuration of nickel (Z = 28) in ground state is 3d 84s2. Nickel in this complex is in the +2 oxidation states. It achieves +2 oxidation state by the loss of the two 4s- electrons. The resulting of Ni2+ ion has outer electronic configuration of 3d8. The two unpaired 3d- electrons are forced to pair up.
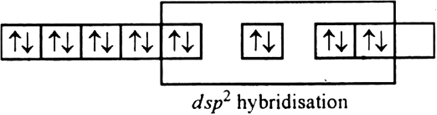
The resulting vacant 3d-orbital along with the 4s-orbital and two 4p-orbitals hybridised to give four equivalent dsp2 hybridised orbitals. Four pairs of electrons, one from each cyanide ion occupy the four vacant hybrid orbitals so produced. Therefore, it is diamagnetic because there is no unpaired electrons.
Discuss the nature of bonding in metal carbonyls.
Carbonyls are formed by the transitional elements because of the presence of vacant d-orbitals. In carbonyl the oxidation state of the metal is zero.
During the formation of carbonyls the vacant d-orbitals of metal are over-lapped by the filled carbon σ-orbitals. This results the formation of σ-bond between metal and C of CO due to the donation of electron pair of carbon into the vacant d-orbitals of metal. On the other hand a -overlap takes place by a back donation of electrons from a filled d-orbitals of metal into the vacant antibonding p-orbitals of CO and a p M → C bond is formed.![]()
Donation of lone pair of electrons by the C of CO into vacant d-orbital of the metal (sigma overlap)
.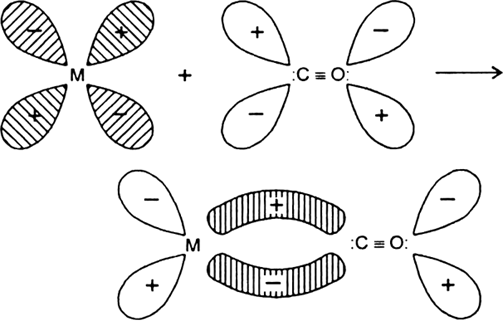
Fig. Donation of electrons from filled d-orbitals of metal into vacant antibonding p - orbital of CO(Pit overlap).
Give the oxidation state, d-orbital occuptaion and coordination number of the central metal ion in the following complexes:
K3[Co(C2O4)3]
The central metal ion is Co..
It coordination number is 6.
The oxidation state can be given as:
X-6 = -3
X= +3
Oxidation state is +3.
d-orbital occupation is since oxalate is strong field ligand therefore pairing occur.
Give the oxidation state, d-orbital occuptaion and coordination number of the central metal ion in the following complexes:
cis-[Cr(en) 2Cl2]Cl
The central metal ion is Cr.
The coordination number is 6.
Ethylene diamine is neutral ligand and chlorine is Monodante ligand thus oxidation is:
X+2(0)+2(-1) = +1
x-2 = +1
x= 3
Oxidation state = + 3
d-orbital occupation is .
Give the oxidation state, d-orbital occuptaion and coordination number of the central metal ion in the following complexes:
(NH4)2[CoF4]
The central metal ion is Co.
The coordination number is 4.
Fluorine is monodante ligand (-1) thus oxidation state is:
x-4 = -2
x =+2
Oxidation state = +2
d-orbital occupation is
Give the oxidation state, d-orbital occuptaion and coordination number of the central metal ion in the following complexes:
[Mn(H2O)6SO4
The central metal ion is Mn.
The coordination number is 6.
Water is neutral ligand thus oxidation state is:
X+0 =+2
X= +2
Oxidation state = +2
d-orbital occupation is
Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration & coordination number, Also give stereochemistry and magnetic moment of the complex:
K[Cr(H2O)2(C2O4)2]. 3H2O
K[Cr(H2O)2(C2O4)2] 3H2O
IUPAC: Potassium diaquadioxalatochromate (III) trihydrate.
Oxidation state = +3
Electronic configuration: 3d3 : t2g3.
sterochemistry : Coordination number = 6
Coordination number = 6
it have three unpaired electron therefore magnetic moment is calculated as :
[n(n+2)]1/2
where n= unpaired electron
[3(3+2)]1/2
[15]1/2 =4.80 B.M
Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration & coordination number, Also give stereochemistry and magnetic moment of the complex:
CrCl3(py)3
CrCl3(py)3
IUPAC name: Trichloridotripyridinechromium(III) oxidation state +3.
Electronic configuration for d3 =t2g3 c
oordination number =6 .
shape : Octahedral.
Sterochemistry:
Stereochemistry both isomers are optically active. Therefore, a total of 4 isomers exist.
Magnetic moment
Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration & coordination number, Also give stereochemistry and magnetic moment of the complex:
Cs[FeCl4].
Cs[FeCl4]
IUPAC name: Caesium tetrachloroferrate (III)
Oxidation state of Fe = +3
Electronic configuration of d6 = eg2 t2g3
Coordination number =4
Shape: tetrahedral
Stereochemistry: optically inactive
Magnetic moment:
What is meant by stability of coordinate compound in solution? State the factors which govern stability of complexes.
The stability of a complex in solution refers to the degree of association between the two species involved in the state of equilibrium. The magnitude of the (stability or formation) equilibrium constant for the association, quantitatively express the stability.
The complex formation is a Lewis acid-base concept.
K = M
Here a+, x– and b+ are the charges on metal, ligand and complex respectively. Charge balance requires that
(a+)+ n(x–) = (b+)
The stability constant; K of the complex can be given as
Higher the value of K, more the stability of the coordination compound.
The stability of the coordination compound depends on:
(i) Nature of the ligand: Chelating ligands form strong and more stable complexes than the monodendate ligands.
The - bond ligands form more stable complexes than the σ bonded complex.
(ii) Nature of the metal atom/ion: Small, highly charged metal ions form more stable complexes than large size, lowly charged metal ion.
What is meant by the chelate effect? Give an example.
The stability which a coordinate compound achieve due to the formation of chelate or ring by a polydendate ligand is called chelate effect.
An example of a chelate ring occurs in the ethylenediamine-cadmium complex:
Discuss briefly the role of coordination compounds in biological systems.
Biological system:
(i) Haemoglobin the red blood cell which acts as oxygen carrier to different parts of the body is a complex of iron(II).
(ii) Vitamin B12 is a complex of cobalt metal. Trace metals also function through coordination processes.
(iii) A complex of zinc(II) called enzyme CPA helps in digestion of food.
(iv) The chlorophyll, the green colouring plant pigment that plays an important role in photosynthesis is a complex of magnesium. In chlorophyll Mg is coordinated to four nitrogens of porphyin units.
Discuss briefly the role of coordination compounds in analytical chemistry.
Analytical chemistry:
(i) Multidentate ligand, EDTA (ethylene diamine tetra acetic acid) forms highly stable complexes with metal ions like Ca2+ and Mg2+. This fact is used to estimate the hardness of water by a simple titration method using EDTA solution.
(ii) A confirmatory test for the detection of copper(II) involves the formation of a deep-blue coloured complex, [Cu(NH3)4]2+, on addition of ammonia solution to a solution of copper(II) salt.
(iii) The separation of group I precipitate of AgCl, Hg2Cl2 and PbCl2 involves in addition of aqueous ammonia solution to the precipitate, when only silver chloride dissolves, due to the formation of the complex ion, [Ag(NH3)2]+
AgCl + 2NH3 → [Ag(NH3)2]+ + Cl–
Hg2Cl2 and PbCl2 do not form complex ions with NH3 and hence, do not dissolve.
(iv) A confirmatory test for nickel consists in adding a solution of dimethyl glyoxime, when a scarlet-red coloured precipitate is formed, due to the formation of a chelate complex. .
Discuss briefly the role of coordination compounds in medicinal chemistry.
Medicinal Chemistry: Many complex compounds are used in medicines. Some common examples are:
(i) Vitamin B12 used to prevent anaemia is a complex compound of cobalt.
(ii) The complex of calcium and EDTA is used in the treatment of lead poisoning. Inside the body calcium in the complex is replaced by lead. The more soluble lead – EDTA complex is eliminated in urine.
(iii) The platinum complex cis-[Pt(NH3)2Cl2] known as cis platin is used in the treatment of cancer.
Discuss briefly the role of coordination compounds in extraction/metallurgy of metals.
Extraction/metallurgy of Metals:
(i) Extraction of metals like silver and gold is carried out by forming their soluble cyanide complex, e.g.,
The solution containing cyanide complex is then treated with zinc, when gold is precipitated.
2[Au(CN)2]– + Zn → [Zn(CN)4]2– + 2Au
(ii) Coordination compounds of silver and gold are used as the constituents of electroplating baths for the controlled delivery of Ag+ and Au ions, during electro-refining of these metals.
What is macrocyclic effect?
How does the metal carbonyls gain stability although CO is a weak donor?
The metal -carbon bond in metal carbonyls posses both σ and π character. The M–C σ bond is formed by the donation of lone pair of electrons on the metal. The M–C π bond is formed by the donation of a pair of electrons from a filled d orbital of metal into the vacant antibonding π* orbital of carbon monoxide. The metal to ligand bonding creates a synergic effect which strengthens the bond between CO and the metal.
[CoF6]3– is paramagnetic whereas [Co(C2O4)3]3– is diamagnetic. Why?
[NiCl4]2– is paramagnetic in nature, explain.
Deduce the magnetic behaviour of each of the following:
(i) [Cr(NH3 )5Cl]2+
(ii) Fe(CO)5 [At. No. Cr = 24, Fe = 26]
It is paramagnetic due to presence to unpaired electrons.
Since it have three unpaired electron therefore magnetic moment will be.
(ii) Fe(26) has outer electronic configuration 4s2,3d6.
Fe(O) has outer electronic configuration 4s°, 3 d8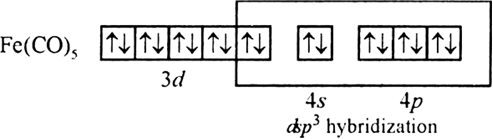
It is diamagnetic because there is no unpaired electron.
Using valence bond theory predict the geometry and magnetic behaviour of [Cr(NH3)6]3+ ion [Cr = 24].
Cr(NH3)6]3+
The outer electronic configuration of Cr is 4s1 3d5.
Its oxidation state is +3. therefor now the electronic configuration of Cr3+ is 4s°3d3.
since it have three number of unpaired electron therefore it is paramagnetic in nature. It has d2sp3 hybridization, octahedral shape
Write IUPAC name of [Pt(NH3)2Cl2].
Using valence bond theory, predict the shape and magnetic character of [Ni(CO)4] [Ni = 28].
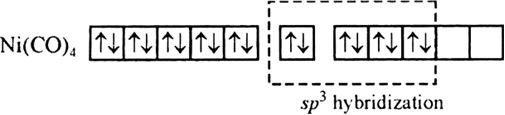
Since there is no any unpaired electron therefore it is diamagnetic in nature and tetrahedral in shape due to sp3 hybridization.
Give one example of application of coordination compounds in medicine.
What type of isomerism is exhibited by the following pair:
[Co(NH3)5Br]SO4 and [Co(NH3)SO4] Br
Give a chemical test to distinguish them.
[Co(NH3)5Br]SO4 and [Co(NH3)SO4] Br exhibit Ionisation isomers.
Above two can be distinguished by chemical test such that [Co(NH3)5(SO4)]Br react with silver ion to precipitate silver bromide, AgBr whereas [Co(NH3)5Br]SO4 does not react with silver ion as a bromide is bonded to cobalt and hence not free to react.
Give one example of co-ordination compounds useful in biological processes.
Under the valence bond approach explain the shape and magnetic behaviour of [Ni(NH3)6]2+.[Given At. No. of Ni = 28]
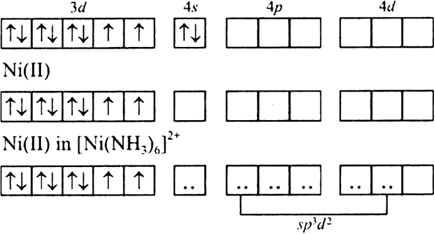
Since there are two unpaired electrons, so the species is paramagnetic and since it is sp3d2 hybridization therefore shape is octahedral.
Explain the following:
Cobalt metal complex is pink when it is octahedral [Co(H2O)6]2+ and it is blue when tetrahedral [CoCl4]2–
Explain the following:
[Ni(H2,O)6]2+ turns blue when changed to [Ni(NH3)6]3+ by adding NH3
Explain the following:
Violet coloured [Cr(H2O)6]3+ becomes bright blue when reduced to [Cr(H2O)6]2+
What do you understand by the term stability constant, K of a complex? Knowing that the value of K for [Cu(NH3)4]2+ is 4.5 x 1011 and for [Cu(CN)4]2– is 2.0 x 1027, suggest
(a) Which complex species will furnish less Cu2+ ions in solution and
(b) Which out of NH3and CN– is a stronger ligand?
where a = charge on the metal ion, x = charge on the ligand and b+ = charge on the complex ion.
Stability constant,
(a)
Evidently, concentration of CN–(aq) ions furnished by [Cu(CN)4]2– complex ion is less than that furnished by [Cu(NH3)4]2+ complex ion.
(b) Since CN– ligand forms more stable complex with Cu2+ ions than NH3 ligand with Cu2+ ions, so CN– ligand is a stronger ligand than NH3.
Illustrate with an example ionization isomerism in coordination compounds.
They give SO42– and Br– respectively in aqueous solution. Violet coloured complex gives white precipitate with BaCl2 solution whereas red coloured complex gives yellow precipitate with AgNO3 solution.
Formation of complex is exothermic or endothermic process. Explain why? What is the effect of temperature on stability of complex compound?
Formation of complex is an exothermic process because there is force of attraction between central metal ion and ligands which breaks and
energy Release and new bond is formed.
Stability of complex decreases with the increase in temperature because formation of complex is exothermic process. On heating, coordinate bond between central metal ion and ligand will break.
Give the IUPAC name of [CrCl2 (H2O)4]Cl
Give the number of unpaired electrons in the following complex ions:
[FeF6]4– and [Fe(CN)6]4–
Number of unpaired electrons in [Fe(CN)6]4– =0
Name the isomerism exhibited by the following pair of coordination compounds:
[Co(NH3)5Br]SO4 and [Co(NH3)5SO4] Br
What is a ligand? Give an example of a bidentate ligand.
The atoms or molecules or ions which donate pair of electrons to the central metal atom and thus forms coordinate bond with the central metal atoms are called ligands.
Example of a bidentate ligand: Ethylene diamine.
Explain as to how the two complexes of nickel, [Ni(CN)4]2– and Ni(CO)4, have different structures but do not differ in their magnetic behaviour (Ni = 28).

In presence of strong field CN- ions, all the electrons are paired up. The empty 4d, 3s and two 4p orbitals undergo dsp2 hybridization to make bonds with CN- ligands in square planar geometry
(ii) Ni(CO)4 : In case the valence shell electronic configuration of ground state Ni atom is 3d8 4s2. All of these 10 electrons are pushed into 3d orbitals and get paired up when strong field CO ligands approach Ni atom. The empty 4s and three 4p orbitals undergo sp3 hybridization and form bonds with CO ligands to give tetrahedral

What is the basis of formation of the spectrochemical series?
Spectrochemical series is a series in which the ligands have been arranged in order of increasing field strength.
I– < Br– <SCN– < Cl– < S2– < F– < OH– < C2O42– < H2O < NCS–< edta4– < NH3 < en < CN– < CO.
Draw the structures of geometrical isomers of the following coordination complexes:
[Co(NH3)3Cl3] and [CoCl2(en)2+]
(en = ethylene diamine and atomic number of Co is 27).
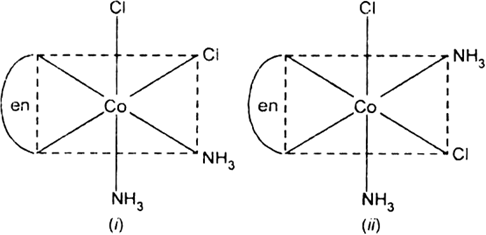
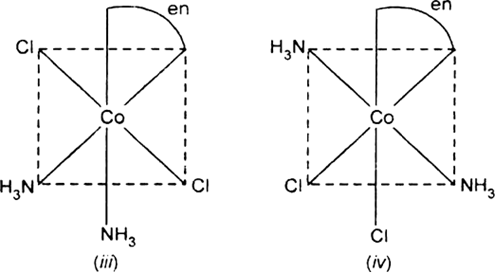
All the four geometric isomers are un-symmetrical and each shows optical isomerism i.e., forms two optical isomers d and l forms which are mirror image of each other in similar way as shown in (a).
[CoCl2(en)2]+: Two geometrical isomers cis-and transforms are possible.
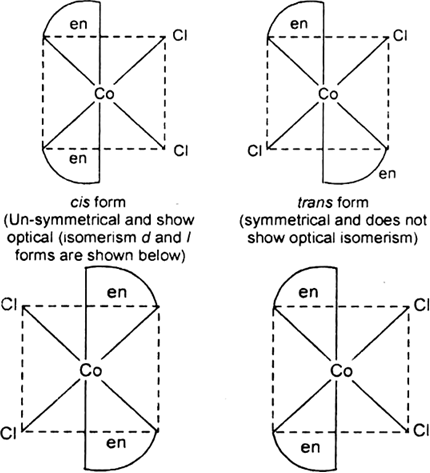
Fig. d and 1 forms of cis [CoCl2(en)2]+.
Explain with examples geometric and optical isomerism.
Geometrical isomerism:
This type of isomerism arises in heteroleptic
complexes due to different possible geometric
arrangements of the ligands. Important examples of this behaviour are found with coordination numbers 4 and 6. In a square planar complex of formula [MX2L2] (X and L are unidentate), the two ligands X may be arranged adjacent to each other in a cis isomer, or opposite to each other in a trans isomer. for example geometrical isomers of Pt[(NH3)2Cl2](3).png)
Each geometrical isomer has a central platinum surrounded by the same four ligands (2 chloro and 2 amine in each case) which lie at the corners of a square. But they differ in the special positions of the ligands. In cis-isomer two similar ligands occupy positions at 90° to one another. In trans-isomer two similar ligands occupy positions opposite to one another at 180° apart.
Optical isomerism: When the coordination compounds have similar formula but differ in their abilities to rotate directions of the plane of the polarized light, they are said to exhibit optical isomerism and the molecules are optical isomers. The optical isomers are pair of molecules which are non-super imposabie mirror images of each other. For example, cis-dichlorobis (ethylene diamine) cobalt(II) ion exhibits optical activity.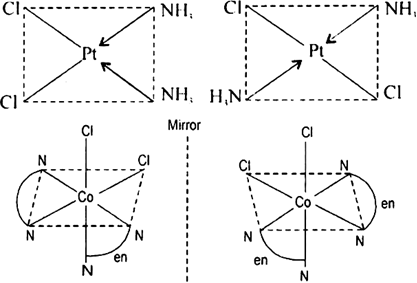
Fig. Cis [Co(en)2Cl2,]+ and its mirror image.
One form of cis[Co(en)2Cl2] which rotates plane of polarized light in right direction is called dextro isomer (or d-form or + isomer). The other isomer which rotates the plane of polarised light in left direction is designated laevo-isomer (or l or-isomer).
Mention the geometrical shapes attained by the following types of hybrid orbitals: (a) sp3, (b) dsp2, (c) d2sp3. Give an example for each.
(a) sp3 hybrid orbitals: This type of hybridization explains the tetrahedral geometry of complexes such as Ni(CO)4 and [Zn(NH3)4]2+.
(b) dsp2 hybrid orbitals: These hybrid orbitals attain square planar shape, dsp2 hybrid orbitals are involved in the bonding of the complexes such as [Ni(CN)4] and [Pt(NH3)4]2+.
(c) d2sp3 hybrid orbitals: These hybrid orbitals attain octahedral shape and explain the bonding in the complexes such as [Co(NH3)6]3+ and [Fe(CN)6]3–.
Illustrate the isomerism:
Coordination isomerism in coordination compounds.
Coordination isomerism: This type of isomerism occurs in compounds containing both cationic and anionic complexes and isomers differ in the distribution of ligands in the coordination sphere of cationic and anionic parts. The examples are:
(i) [Co(NH3)6] [Cr(CN)6] and [Cr(CNH3)6] [Co(CN)6]
(ii)[Cu(NH3)4] [PtCl4] and [Pt(NH3)4] [CuCl4]
This type of isomerism is also shown by compounds in which the metal ion is the same in both cationic and anionic complexes. For example:
(a) [Cr(NH3)6] [Cr(CN)6] and [Cr(NH3)2(CN)4][Cr(NH3)2(CN)4]
(b) [Pt(NH3)4] [PtCl4] and [PtCl(NH3)3] [PtCl3(NH3)]
A metal ion Mn+ having d4 valence electronic configuration combines with three didendate ligands to form complex compound. Assuming ∧0 > P.
(i) Draw the diagram showing d-orbital splitting during this complex formation.
(ii) Write the electronic configuration of the valence electrons of the metal Mn+ in terms to t2g, and eg .
(iii) What type of hybridisation will Mn+ ion have?
(iv) Name the type of isomerism exhibited by this complex.
(i) Since we assuming ∧0 > P thus, ligand is weak field ligand therefore it does not caused pairing. 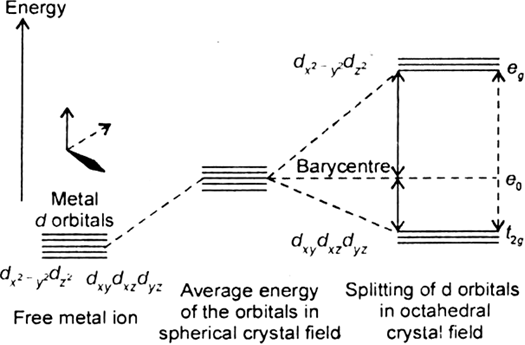
(ii) Electronic configuration is t32g, e1g because ligand is weak.
(iii) ligand is three didendate ligand therefore it contribute six electron thus it hybirdization is sp3d2
Illustrate with an example of the following:
Linkage isomerism
Linkage isomerism: The compounds which have the same molecular formula but differ in the mode of attachment of a ligand to the metal atom or ion are called linkage isomers. For example, in NO2– ion, the nitrogen atom as well as the oxygen atom can donate their lone pairs. This gives rise to isomerism. If nitrogen donates its lone pair, one particular compound will be formed. On the other hand, if oxygen donates its lone pair, a different compound is obtained. If the bonding is through N, the ligand is named as nitro and if it is through O, it is named as nitrito.
NO2– nitro ONO– nitritol
For example, two diffeent penta-amine cobalt(III) chlorides each containing the NO2 group in the complex ion have been prepared. These are:
(i) Explain geometrical isomerism with reference to square planar complexes giving one example. How is tetrahedral complexes with simple ligands do not exhibit geometrical isomerism?
(ii) Using valence bond theory, predict the shape and magnetism (paramagnetism) or diamagnetism of [Co(CO)4]– (at. no. of Co = 27)?
(iii) How is stability of coordination compounds determined in aqueous solution?
(i)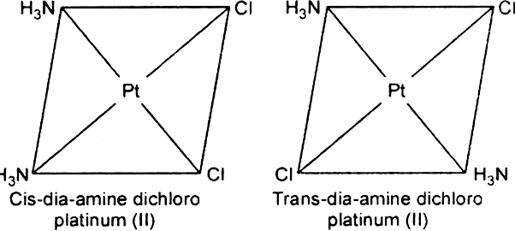
Tetrahedral complexes do not show geometrical isomerism because relative position of ligands are the same.
(ii) Co (27) = 4s23d 7
Co3+ = 4s°3d 6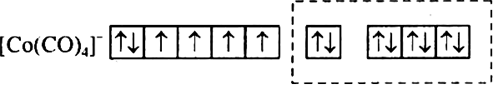
It has sp3 hybridisation, it is paramagnetic.
(iii) Stability of coordination compound in aqueous solution is determined with the help of stability constant. Higher the value of stability constant, greater will be stability. Smaller the cation, higher the charge on the cation, more stable will be the complex.
The number of ligands attached to the central atom is termed the ___________ of the central atom.
coordination number
The total number of electrons on the central atom including those gained by bonding is called the ___________.
State whether the following statements are True or False:
A.
Trien is an example of terdentate ligand.B.
The IUPAC name of [PtCl2(NH2CH2CH2 NH2)2](NO3), is dichloro bis (1, 2-ethane diamine) plaiinum (IV) dinitrate.C.
NH3 and H2O both are monodentate ligands.D.
Chelating ligands form more stable complexes than monodentate ligands.E.
Transition metal ions form complexes because they have empty orbitals.F.
A bidentate ligand has 3 coordination sites.G.
Tetrahedral complexes do not have geometric isomers.H.
The IUPAC name of [CuCl4]2– is tetrachloro cuprate(II).B. FALSE
C. TRUE
D. TRUE
E. TRUE
F. FALSE
G. TRUE
H. FALSE
Which of the following complex will not show colour?
- K3[VF6]
- [Cr(NH3)6]Cl3
- [Sc(H2O)6]3+
- [NiCl4]2–
C.
[Sc(H2O)6]3+A group of atoms can function as a ligand only when
It is a small molecule
It has an unshared electron pair
It is negatively charged ion
It is positively charged ion.
B.
It has an unshared electron pair
Among the following the most stable complex is - [Fe(H2O)6]3+
[Fe(NH3)6]3+
- [Fe(C2O4)3]3–
- [FeCl6]3–
[Fe(NH3)6]3+
C.
[Fe(C2O4)3]3–Which complex has square planar structure?
- Ni(CO)4
- [NiCl4]2-
[Ni(CN)4]2–
- [Cu(NH3)4]2+
C.
[Ni(CN)4]2–
What type of geometrical shape the following types of hybrid orbitals have? (a) d2sp3, (b) dsp2.
The geometrical shape of the given hybird orbital is given according to valence bond theory :
a) d2sp3 = Octahedral geometry
b) dsp2 = Square planar
Write IUPAC name of of [Co(NO2)(NH3)5]Cl2.
The IUPAC name of the coordination compound is pentaammine nitrito-N-cobalt (III) chloride
What is the coordination number of cobalt in [Co(en)2H2OBr]Cl?
coordination number of cobalt is 6.
Write the formula of coordination compound: Pentammine chloro cobalt(III) chloride.
[Co(NH₃)₅Cl]Cl₂.
What is the oxidation state of platinum in [Pt(en)2Cl2]?
Oxidation state of platinum
Pt = x
charge of ethylene diamine is (en)= 0
charge of chlorine is Cl=(-1)
thus,
x+0+(-1)x2 =0
x+(-2) =0
x=2
Why is [Cr(NH3)6]3+ ion not diamagnetic?
Electronic configuration of Cr is 4s1 3d5 . the oxidation number of Cr is in [Cr(NH3)6]3+ is Cr3+. therefore,

Six pairs of electrons, one from each NH3 molecule, occupy the six hybrid orbitals. Thus, the complex has octahedral geometry. due to unpaired electrons the complex is paramagnetic.
What is the type of hybridization associated with Cu2+ ion in [Cu(NH3)4]2+ complex?
4- Coordinate complex will be tetrahedral or square planar. In complex [Cu(NH3)4]2+. According to VBT the complex will tetrahedral.
The hybrization of the complex is dsp2.
In a geometry of [PtCl4]2– is square planar, what orbitals of platinum are involved in the bonding?
The electronic configuration of Platinum is [Xe] 4f14 5d9 6s1
Or [Xe] 4f14 5d8 6s2
Oxidation of Pt in this complex is +2 thus, 
The four chlorine atom filled the empty orbital. Therefore the the hybridziation of [PtCl4]2– is dsp2.
Complex should be tetrahedral instead of square planar theoretically. But the size of Pt is large that it forms strong bond with ligand. Due to which strong repulsion between the electron of Pt and ligand takes place which result in strong crystal field splitting. The strong field splitting breaks the degeneracy of dx2- y2 and dz2 orbital. Hence stabilizes the square planer arrangement more than tetrahedral thus it should be square planar.
What is the oxidation state of metal in metal carbonlys?
CO is a neutral molecule that is it carries no charge
for oxidation state to exist for the metal.
Identify the ligands in complex ion [Co(en)2Cl(ONO)]+ and write its IUPAC name.
ligand are:
en = ethylene diamine
Cl= chloride
ONO= nitrito
its IUPAC name is chlorobis(ethylenediamine)nitritocobalt(III)ion.
Give an example of hexadentate ligand.
EDTA4- (ethylene diamine tetracetate ion) is hexadentate ligand as it can bind through two nitrogen and four oxygen atoms to central metal ion.

structure of EDTA4-
What is coordination number of metal ion in [Pt(NH3)2(C2O4)]?
In this complex the coordination number of metal ion is 4. such to two electron pair from diamine and 2 electron pair from oxalate (didentate ligand).
How would you account for the fact that both Ni(CO)4 and (NiCl4)2– have a tetrahedral geometry? (Atomic number of Ni = 28). Which one has a higher magnetic moment?
The valence shell electronic configuration of ground state Ni atom is 3d8 4s2.
All of these 10 electrons are pushed into 3d orbitals and get paired up when strong field CO ligands approach Ni atom. The empty 4s and three 4p orbitals undergo sp3 hybridization and form bonds with CO ligands to give Ni(CO)4. Thus Ni(CO)4 is diamagnetic.
in NiCl42-, there is Ni2+ ion, However, in presence of weak field Cl- ligands, no pairing of d-electrons occurs. Therefore, Ni2+ undergoes sp3 hybridization to make bonds with Cl- ligands in tetrahedral geometry. As there are unpaired electrons in the d-orbitals, NiCl42- is paramagnetic
Since (NiCl4)2– is paramagnetic thus it have more magnetic moment.
What do you understand by crystal field splitting? Draw figure to show splitting of degenerate d-orbitals in an octahedral crystal field.
The crystal field splitting, depends upon the field produced by the ligand and charge on the metal ion. Some ligands are able to produce strong fields in which case, the splitting will be large whereas others produce weak fields and consequently result in small splitting of d orbitals. Therefore the d orbital will be degenrate in two set such as t2g and eg orbital .
What are the main postulates of Werner’s theory? Write the correct formula and IUPAC name of the coordination compound for CrCl3.6H2O, one mole of which give two moles of AgCl with AgNO3,.
The main postualtes of werner's theory:
1. In coordination compounds metals show two types of linkages (valences)-primary and secondary.
2. The primary valences are normally ionisable and are satisfied by negative ions.
3. The secondary valences are non ionisable. These are satisfied by neutral molecules or negative ions. The secondary valence is equal to the coordination number and is fixed for a metal.
4. The ions/groups bound by the secondary linkages to the metal have characteristic spatial arrangements corresponding to different
coordination numbers.
In modern formulations, such spatial arrangements are called coordination polyhedra. The species within the square bracket are coordination entities or complexes and the ions outside the square bracket are called counter ions.
The correct formula of CrCl3.6H2O is [Cr(H2O)6]Cl3 IUPAC name is hexaaquachromium(lll)chloride.
one mole of [CrCl3.H2O5] give two moles of AgCl with AgNO3.
[Ni(CO)4 has a tetrahedral geometry whereas [Ni(CN)4]4– has square planar geometry. Why?
Ni(CO)4 = Ni + 4CO
The valence shell electronic configuration of ground state Ni atom is 3d8 4s2.
All of these 10 electrons are pushed into 3d orbitals and get paired up when strong field CO ligands approach Ni atom. The empty 4s and three 4p orbitals undergo sp3 hybridization and form bonds with CO ligands to give Ni(CO)4. Thus Ni(CO)4 is diamagnetic.
thus according to VBT sp3 hybridization have tetrahedral geometry.
In [Ni(CN)4]2-, there is Ni2+ ion for which the electronic configuration in the valence shell is 3d8 4s0.
In presence of strong field CN- ions, all the electrons are paired up. The empty 4d, 3s and two 4p orbitals undergo dsp2 hybridization to make bonds with CN- ligands in square planar geometry. Thus [Ni(CN)4]2- is diamagnetic.

[Fe(CN)6]4– is diamagnetic while [FeF6]4– is strongly paramagnetic. Why?
[Fe(CN)6]4− In the above coordination complex, iron exists in the +II oxidation state. Fe2+. Electronic configuration of Fe2+is 4s0 3d6.
As CN− is a strong field ligand, it causes the pairing of the unpaired 3d electrons.
Since there are six ligands around the central metal ion, the most feasible hybridization is d2sp3. d2sp3 hybridized orbitals of Fe2+ . 6 electron pairs from CN− ions occupy the six hybrid d2sp3orbitals. Then,
Since CN- is strong field ligand it cause pairing. hence it is diamagnetic.
In this complex, the oxidation state of Fe is +3. eletronic configuration is 4s0 3d5.
There are 6 F− ions. Thus, it will undergo d2sp3 or sp3d2 hybridization. As F− is a weak field ligand, it does not cause the pairing of the electrons in the 3d orbital. Hence, the most feasible hybridization is sp3d2.
Since [FeF6]4– have unpaired electrons. Hence it is strongly paramagnetic.
What is complex? How is it different from a double salt?
Double salts as well as complexes are formed by the combination of two or more stable compounds in stoichiometric ratio. However, they differ in the fact that double salts such as carnallite, KCl.MgCl2.6H2O,
Mohr’s salt, FeSO4.(NH4)2SO4.6H2O, potash alum, KAl(SO4)2.12H2O, et,.dissociate into simple ions completely when dissolved in water. However, complex ions such as [Fe(CN)6]4– of K4Fe(CN)6, do not dissociate into .
Give the systematic name for each of the following compounds:
(i)[Pt(NH3)4Cl2][PtCl4]
(ii) [Cr(CO)5Cl](ClO3)2
(iii) K3[Cr(CN)6]
(iv)[Co(NH)4Cl2]+
(v) (NH4)3[Co(NO2)6]
(vi) K2[CuCl4]
(i) Tetraamminedichloroplatinum(IV) tetrachloroplatinate(II)
(ii) Pentacarbonylchlorochromium(III) chlorate
(iii) Potassium hexacyanochromate(III)
(iv) dichlorotetraminocobalt(III) ion
(v) Ammonium cobalti- nitrite
(vi) Potassium Tetrachlorocuprate(II)
Give the chemical formula for each of the following compounds:
(i) Potassium hexanitro cobaltate(III).
(ii) Potassium hexacyano cobaltate(III).
(iii) Ammonium trans-dichlorodiodo aurate(III).
(iv) Sodium pentacyano carbonyl ferrate(II).
(v) Diamminechlorido(methylamine)plantinum(II)
chloride.
(vi)Dichloridobis(ethane–1,2diamine)platinum(IV) nitrate
i) K3[Co(NO2)6]
ii)K3[Co(CN)6]
iii)(NH3)2[AuCl2I2]
iv)Na2[Fe(CN5)NO]
v) [Pt(NH3)2Cl(NH2CH3)]Cl
vi) PtCl2[(en)2](NO3)2
Explain the geometry of [Cr(NH3)6]3+ ion by valence bond theory of complexes. Why is this not diamagnetic?
Electronic configuration of Cr is 4s1 3d5. In the complex [Cr(NH3)6]3+ Cr is in the +3 oxidation state i.e., d3 configuration. Also, NH3 is a weak field ligand that does not cause the pairing of the electrons in the 3d orbital.
Therefore, it undergoes d2sp3 hybridization and the electrons in the 3d orbitals remain unpaired. Hence, it is paramagnetic in nature.
Explain the geometry of Ni(CO)4 by valence bond theory. Why is this molecule diamagnetic? (Atomic number of Ni = 28).
Ni(CO)4 = Ni + 4CO
The valence shell electronic configuration of ground state Ni atom is 3d8 4s2.
All of these 10 electrons are pushed into 3d orbitals and get paired up when strong field CO ligands approach Ni atom. The empty 4s and three 4p orbitals undergo sp3 hybridization and form bonds with CO ligands to give Ni(CO)4. Thus Ni(CO)4 is diamagnetic.
Using valency bond approach predict the shape and magnetism (paramagnetic or diamagnetic) of [Ni(CN)4]–.
In [Ni(CN)4]2-, there is Ni2+ ion for which the electronic configuration in the valence shell is 3d8 4s0.
In presence of strong field CN- ions, all the electrons are paired up. The empty 4d, 3s and two 4p orbitals undergo dsp2 hybridization to make bonds with CN- ligands in square planar geometry. Thus [Ni(CN)4]2- is diamagnetic.

Using valence bond approach, predict the shape and magnetism (paramagnetic or diamagnetic) of [Ni(CO)4].
The valence shell electronic configuration of ground state Ni atom is 3d8 4s2.
All of these 10 electrons are pushed into 3d orbitals and get paired up when strong field CO ligands approach Ni atom. The empty 4s and three 4p orbitals undergo sp3 hybridization and form bonds with CO ligands to give Ni(CO)4. Thus Ni(CO)4 is diamagnetic.
Since hybirdization of Ni(CO)4 is sp3 therefore according to VBT it has tetrahedral shape.
Explain the following terms: (i) Inner orbital complex and (ii) outer orbital complex.
i) Inner orbital complexes that use inner d- orbitals in hybirdisation; for example [Co(NH3)]3+ is inner orbital because the complex is using inner d-orbital therefore it’s hybridization is d2sp3. Also know as low spin complex.
ii) Outer-orbital:complex that is use outer d- orbitals in hybirdisation; for example [CoF6]3- uses outer orbital (4d ) in hybridisation (sp3d2). It is thus called outer orbital or high spin or spin free complex.
What is meant by stability constant of a complex?
The stability of a complex in solution refers to the degree of association between the two species involved in the state of equilibrium. The magnitude of the equilibrium constant for the association, quantitatively expresses the stability.
Define cis-trans isomerism. Draw the cis and trans isomers of [Co(NH3)3Cl3) ion.
Cis-trans isomerism are also known as geometric isomerism or configurational isomerism.
Deduce the structures of [NiCl4]2– and [Ni(CN)4]2– considering the hybridization of the metal ion. Calculate the magnetic moment (spin only) of the species.
NiCl42-, there is Ni2+ ion, However, in presence of weak field Cl- ligands, NO pairing of d-electrons occurs. Therefore, Ni2+ undergoes sp3 hybridization to make bonds with Cl- ligands in tetrahedral geometry. As there are unpaired electrons in the d-orbitals, NiCl42- is paramagnetic. 
Since it have two unpaired electron electron therefore the magnetic moment :
In [Ni(CN)4]2-, there is Ni2+ ion for which the electronic configuration in the valence shell is 3d8 4s0.
In presence of strong field CN- ions, all the electrons are paired up. The empty 4d, 3s and two 4p orbitals undergo dsp2 hybridization to make bonds with CN- ligands in square planar geometry. Thus [Ni(CN)4]2- is diamagnetic. 
Since there is no any unpaired electron therefore its magnetic moment is zero.
Describe briefly the nature of bonding in metal carbonyls.
The carbonyls are formed by most of the transition metals. These carbonyls have simple, well defined structures.
The metal-carbon bond in metal carbonyls
possess both σ and π character. The M–C σ bond is formed by the donation of lone pair of electrons on the carbonyl carbon into a vacant orbital of the metal. The M–C π bond is formed by the donation of a pair of electrons from a filled d orbital of metal into the vacant antibonding π* orbital of carbon monoxide. The metal to ligand bonding creates a synergic effect which strengthens the bond between CO and the metal.
Illustrate with an example each of the following terms: (i) Ionization isomerism, (ii) coordination isomerism, (iii) Linkage isomerism, (iv) Geometrical isomerism (v) Optical isomerism.
ii) Coordination isomers: This type of isomerism arises from the interchange of ligands between cationic and anionic entities of different metal ions present in a complex. An example is provided by [Co(NH3)6][Cr(CN)6], in which the NH3 ligands are bound to Co3+ and the CN– ligands to Cr3+. In its coordination isomer [Cr(NH3)6][Co(CN)6], the NH3 ligands are bound to Cr3+ and the CN– ligands to Co3+.
iii) Linkage isomerism: Linkage isomerism arises in a coordination compound containing ambidentate ligand. A simple example is provided by complexes containing the thiocyanate ligand, NCS–, which may bind through the nitrogen to give M–NCS or through sulphur to give M–SCN. For example [Co(NH3)5(NO2)]Cl2, which is obtained as the red form, in which the nitrite ligand is bound through oxygen (–ONO), and as the yellow form, in which the nitrite ligand is bound through nitrogen (–NO2).
iv) Geometrical Isomerism: Geometrical isomerism arises in heteroleptic complexes due to different possible geometric arrangements of the ligands. Important examples of this behaviour are found with coordination numbers 4 and 6. In a square planar complex of formula [MX2L2] (X and L are unidentate), the two ligands X may be arranged adjacent to each other in a cis isomer, or opposite to each other in a trans. For example Cis -trans isomer of [Co(NH3)4Cl2]+.

v) Optical isomerism: Optical isomerism are mirror images that cannot be superimposed on one another. These are called as enantiomers. The molecules or ions that cannot be superimposed are called chiral. The two forms are called dextro (d) and laevo (l) depending upon the direction they rotate the plane of polarised light. For example [Co(en)3]3+.

Write the correct formulae for the following coordination compounds:
(a) CrCl3.6H2O (violet, with 3 chloride ions/unit formula)
(b) CrCl3.6H2O (light green, with 2 chloride ion/unit formula)
(c) CrCl3.5H2O (dark green, with 1 chloride ion/unit formula)
b)[CrCl2(H2O)4]Cl.2H2O
c)[CrCl(H2O)5Cl]H2O
Explain the following terms:
(a) coordination number
(b) counter ions.
a) coordination number : The coordination number of a metal ion in a complex can be defined as the number of ligand donor atoms to which the metal is directly bonded. For example, in the complex ions, [PtCl6]-2 and [Ni(NH3)4]2+, the coordination number of Pt and NI are 6 and 4 respectively.
b) Counter ion: The species with in the square bracket are coordination entities or complexes and the ions outside the square bracket are called counter ions.
A coordination compound has the formula CoCl3.4NH3. It does not liberate ammonia but forms a precipitate with AgNO3. Write the structure and IUPAC name of the complex compound.
The oxidation state of Co in this complex is +3 and that its coordination number is 6. Since, it does not liberate ammonia, the 4 NH3 groups are coordinated to Co. In order to satisfy the coordination number of Co, two chlorides are also coordinated. Therefore, the formula of the complex compounds is [Co(NH3)Cl2]Cl and its IUPAC name is 4 tetraamminodichlorocobalt(III) chloride.
Draw the structure of the following compounds:
(a) cis-[CrCl2(OX)2]3– (b) [Ni(CN)4]2–
(c) [Fe(CN)6]3– (d) Meridional [CrCl3(NH3)3]+
(e) trans-[PtCl2(en)2]2+.

ii) [Ni(CN)4]2–

iii) [Fe(CN)6]3–

iv) Meridional [CrCl3(NH3)3]+

v) trans-[PtCl2(en)2]2+

Write down the IUPAC name of the following complex:
[Cr(NH3)2Cl2(en)]Cl (en = ethylenediamine)
Cr(NH3)2Cl2(en)]Cl
IUPAC Name: Diamminedichlorideethyldiamine chromium (III) chloride
Write the formula for the following complex:
Pentaamminenitrito-o-Cobalt (III)
Formula: [Co(NH3)5(ONO)]2+
Draw the geometrical isomers of complex [Pt(NH3)2Cl2]?
Geometrical isomers of complex [Pt(NH3)2Cl2]
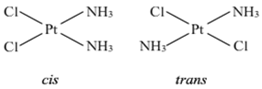
On the basis of crystal field theory, write the electronic configuration for d4 ion if ∆o < P ?
On the basis of crystal field theory, for a d4 ion, if ∆o< P, then the complex is a high spin complex formed by the association of weak field ligands with the metal ion. As a result, the fourth electron enters one of the eg orbitals, thereby, exhibiting the electronic configuration t2g3 eg1.
Write the hybridization and magnetic behaviour of the complex [Ni(CO)4].
(At.no. of Ni = 28)
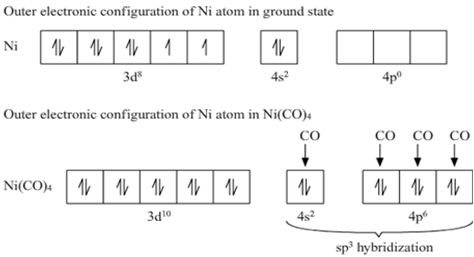
Carbonyl, CO being a strong field ligand causes the pairing of up valence electrons in the Ni atom against the Hund's Rule of Maximum Multiplicity. This results in the formation of an inner orbital complex, [Ni(CO)4] having diamagnetic character. [Ni(CO)4] has sp3 hybridization.
Which of the following is a more stable complex and why?
(i) [Co(NH3)6]3+
(ii) [Co(en)3]3+
Chelating ligands form more stable complexes compared to non-chelating ligands. Since ethylene diamine is a bidentate ligand and forms stable chelate, [Co(en)3]3+ will be a more stable complex than [Co(NH3)6]3+
Write the IUPAC name of the complex [Cr(NH3)4Cl2]+. What type of isomerism does it exhibit?
The IUPAC name of the complex [Cr(NH3)4Cl2]+ is Tetraamminedichlorochromium(III) ion.
This complex exhibits geometrical isomerism. [Cr(NH3)4Cl2]+ is a [MA4B2] type of complex, in which the two chloride ligands may be oriented cis and trans to each other.
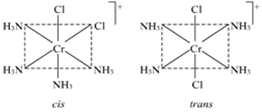
Write the IUPAC names of the following coordination compounds:
(i) [Cr(NH3)3Cl3]
(ii) K3[Fe(CN)6]
(iii) [CoBr2(en)2]+, (en = ethylenediamine)IUPAC Names:
(i) Triamminetrichlorochromium (III)
(ii) Potassium hexacyanoferrate (III)
(iii) Dibromidobis (ethane-1, 2-diammine) cobalt (III) ion
Give the formula of each of the following coordination entities:
(i) Co3+ Ion is bound to one Cl-1 , one NH3 molecule and two bidentate ethylenediamine (en) molecules.
(ii) Ni2+ ion is bound to two water molecules and two oxalate ions. Write the name and magnetic behavior of each of the above coordination entities.
(At. No. Co = 27, Ni = 28)
i) [Co(NH)3(en)2Cl]2+
Amminechloridobis (ethane-1, 2-diamine) cobalt (III) ion.
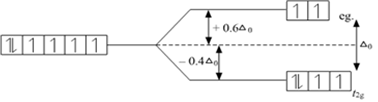
Number of unpaired electrons = 4
So the complex is paramagnetic.
ii) [Ni(H2O)2(C2O4)]2-
Diaquadioxalatonickelate (II) ion
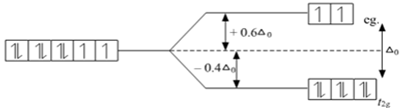
Number of unpaired electrons = 2
So the complex is paramagnetic
Write the name, stereochemistry and magnetic behavior of the following:
(At. nos. Mn = 25, Co = 27, Ni = 28)
(i) K4[Mn (CN)6]
(ii) [Co (NH3)5Cl]Cl2
(iii) K2[Ni (CN)4]
(i) K4 [Mn (CN)6]
Name: Potassium hexacyanomanganate (II)
Stereochemistry - Does not show geometric or optical isomerism
Magnetic behaviour - Paramagnetic
(ii) [Co (NH3)5Cl]Cl2
Name-Pentaamminechloridocobalt (III) Chloride
Stereochemistry- Does not geometric isomerism but is optically active
Magnetic behavior- Paramagnetic
(iii) K2[Ni (CN)4]
Name: Potassium tetracyanonickelate (II)
Stereochemistry -Does not show geometric or optical isomerism
Magnetic behaviour - Diamagnetic
Write down the IUPAC name of the complex [Pt(en)2Cl2]2+. What type of isomerism is shown by this complex?
Complex: [Pt(en)2Cl2]2+
IUPAC name: Dichloridobis(ethane-1,2-diammine)platinum(IV) ion
Isomerism: Geometrical isomerism

Using IUPAC norms write the formulate for the following coordination compounds:
(i) Hexaamminecobalt (III) chloride
(ii) Potassium tetrachloridonickelate (II)
|
IUPAC Names |
Formulae of Complexes |
|
Hexaamminecobalt(III) chloride |
[Co(NH3)6]Cl3 |
|
Potassium tetrachloridonickelate(II) |
K2[NiCl4] |
(a) Write the hybridization and shape of the following complexes:
(i) [CoF6]3–
(ii) [Ni (CN)4]2–
(Atomic number: Co = 27, Ni = 28)
(b) Out of NH3 and CO, which ligand forms a more stable complex with a transition metal and why?
(a)
(i) The atomic number of Co is 27 and its valence shell electronic configuration is 3d74s2.
Co is in +3 oxidation state in the complex [CoF6]3-.
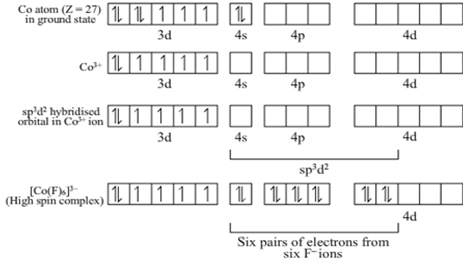
Hence, [CoF6]3- is sp3d2 hybridized and it is octahedral in shape.
(ii) The atomic number of Ni is 28 and its valence shell electronic configuration is 3d84s2.
Ni is in +2 oxidation state in the complex [Ni(CN)4]2–.
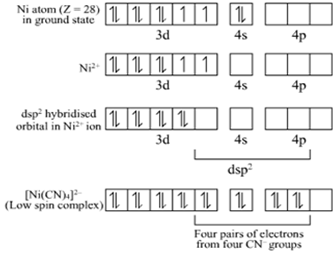
Hence, [Ni(CN)4]2– is dsp2 hybridized and it is square planar in shape.
(b) Out of NH3 and CO, CO ligand forms a more stable complex with transition metal because the metal- carbon bonds in metal carbonyls have both and characters .A bond is formed when carbonyl carbon donates a lone pair of electrons to the vacant orbital of the metal. A bond formed by the donation pair of electrons from filled metal d orbital into the vacant anti- bonding orbital ( also known as back bonding of the carbonyl group). And sigma bond strengthen the pi bond vice-versa. Thus, a synergic effect s created due to this metal- ligand bonding. These synergic effects strengthen the bond between CO and the metal.
For the complex [NiCl4]2-, write
(i) The IUPAC name.
(ii) The hybridization type.
(iii) The shape of the complex.
(Atomic no. of Ni = 28)
[NiCl4]2-
(i) IUPAC name = Tetrachloronickelate (II) ion
(ii) Hybridization of Ni in the complex [NiCl4]2- is sp3. The hybridisation scheme is shown in the following diagram.
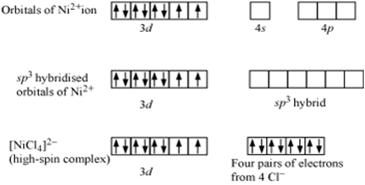
Hence the hybridisation of Ni2+ in the complex is sp3
(iii) As the hybridization of Ni is sp3 so, shape of the complex is tetrahedral.
What is meant by crystal field splitting energy? On the basis of crystal field theory, write the electronic configuration of d4 in terms of t2g and eg in an octahedral field when
(i) ![]() 0 > P
0 > P
(ii) ![]() 0 < P
0 < P
Crystal Field Splitting Energy: Crystal field theory was given to explain the structure and stability of the coordination complexes. This theory has some assumption like the metal ion is considered to be a point positive charge and the ligands are negative charge. The complexes are formed mainly by the d- block elements due to their variable oxidation states and variable coordination number. The d- subshell has 5 degenerate orbitals. when the ligand approach to the metal ion, the energy of the degenerate orbitals is increased and further splitting of degenerate orbital takes place into t2g and eg orbital. The difference between the t2g and eg orbitals is called as the crystal field splitting energy.
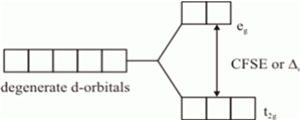
This splitting energy depends on the typo of ligand.
Strong field ligand will have high splitting energy and weak field ligand have low splitting energy.
For electric configuration of d4
(i) When 0 > P
In strong field ligand, the fourth electron will come back and pair in the t2g orbitals. So, the configuration will be Electronic configuration is

(ii) When o < P
In weak field ligand, the Electronic configuration is

Name of the following coordination entities and describe their structure:
(i) [Fe (CN)6]4-
(ii) [Cr (NH3)4Cl2]+
(iii) [Ni (CN) 4]2-
(Atomic numbers Fe = 26. Cr = 24, Ni = 28(i) [Fe (CN)6]4-
IUPAC name: Hexacyanoferrate (II)
Structure: Oxidation state of iron is + 2
Fe2+: Electronic configuration is 3d6 4s° 4p°
Orbitals of Fe2+ ions:
![]()
As CN- is a strong field ligand, it causes the pairing of unpaired 3d electrons
![]()
Since there are six ligands around the central metal ion, the most feasible hydrization is d2sp3.
d2sp2 hybridized orbitals of Fe2+ are:
![]()
6 electron pairs from CN-1 ions occupy the six hybrid d2sp3 orbitals
Then,

Hence, the structure of [Fe (CN)6]4- is octahedral.
(ii) [Cr (NH3)4Cl2] +
Name: Tetraamminedichlorido chromium (III)
Electronic configuration of Cr: 3d4 4s2
Electronic configuration of Cr3+: 3d3
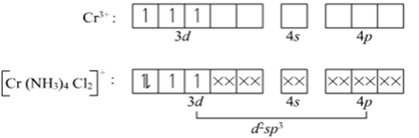
Structure is octahedral with d2sp3 hybridization.
(iii) [Ni (CN) 4]2-
Name: Tetracyanonickelate (II)
Structure: Here oxidation state of Ni is + 2
Ni + 2: Electronic configuration is 3d8 4s0 4p0
Orbitals of Ni2+ ion
![]()
As CN-1is a strong field ligand, it causes the pairing of unpaired 3d electrons.
![]()
Since, there are four ligands around the central metal ion, the most feasible hybridization is dsp2
![]()
4 electron pairs from CN-1 ions occupy the four hybrid dsp2 orbitals
Then,
Hence, the structure of [Ni(CN)4]2- is square planar.
On adding NaOH to ammonium sulphate, a colourless gas with pungent odour is evolved, which forms a blue-coloured complex with Cu2+ ion. Identify the gas.
On adding NaOH to ammonium sulphate, ammonia gas is evolved. It has a pungent odour and forms a blue-coloured complex with the Cu2+ ion. The chemical reactions are as:
![]()
When a co-ordination compound CrCl3.6H2O is mixed with AgNO3, 2 moles of AgCl are precipitated per mole of the compound. Write
(i)Structural formula of the complex.
(ii)IUPAC name of the complex.
When a coordination compound CrCl3.6H2O is mixed with AgNO3 it gives tow moles of AgCl, therefore, the structural formula would contain two Cl− ions satisfying the primary valencies, while five H2O molecules and one Cl− ion are present in the coordination sphere, making the coordination number 6. One H2O molecule will be present as the molecule of hydration.
Structural formula: [CrCl(H2O)5]Cl2.H2O
IUPAC name of the compound: Pentaaquachloridochromium(III) chloride
(a) For the complex [Fe(CN)6]3–, write the hybridization type, magnetic character and spin nature of the complex. (At. number : Fe = 26).
(b) Draw one of the geometrical isomers of the complex [Pt(en)2Cl2]2+ which is optically active.
The electronic configuration of Fe is Ar[18] 4s2 3d6
The electronic configuration of Fe3+ is Ar[18]3d5 4s0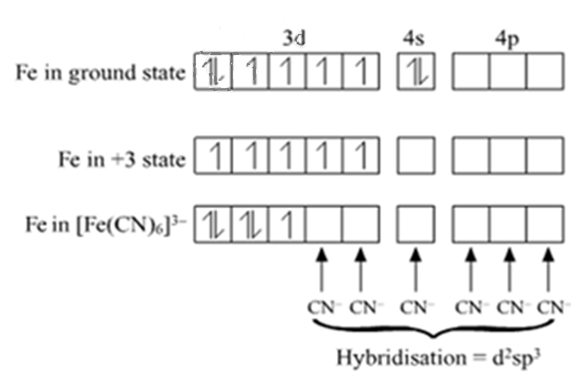
Hybridisation: d2sp3
Magnetic character: Paramagnetic
Spin nature of complex: Low-spin complex
(b) cis-isomer of [Pt(en)2Cl2]2+ is optically active.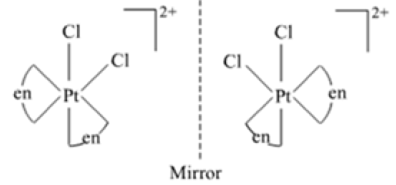
When coordination compound CoCl3.6NH3 is mixed with AgNO3, 3molesof AgCl is precipitated per mole of the compound. write
i) Structural formula of the complex
ii) IUPAC name of the complex
(i) [Co (NH3)6] Cl3
(ii) Hexaamminecobalt(III) chloridea) For the complex [Fe(H2O)6]3+, write the hybridization,magnetic character and spin of the complex.(At.number: Fe=26).
b) Draw one of the geometrical isomers of the complex [Pt(en)2Cl2]2+ which is optically inactive.
i) Fe exists in the +3 oxidation state i.e, in d5 configuration.Since water is the weak ligand, therefore, there is no paired electron. Hence it is strongly paramagnetic and high spin.
Hybridization sp3d2.
ii) 
Write the state of hybridization, the shape and the magnetic behaviour of the following complex entities:
(i) [Cr (NH3)4 Cl2] Cl
(ii) [Co (en) 3] Cl3
(iii) K2 [Ni (CN) 4](i) [Cr (NH3)4 Cl2]Cl
Name - Tetraamminedichloridochromium (III) chloride
Stereochemistry - Shows geometric isomerism and the cis form is optically active
Magnetic behaviour - Paramagnetic
(ii) [Co (en3)]Cl3
Name -tris (ethane-1, 2-diamine) cobalt (III) chloride
Stereochemistry -Shows optical isomerism
Magnetic behaviour - Paramagnetic
(iii) K2 [Ni (CN) 4]
Name -Potassium tetracyano nickelate (II)
Stereochemistry - Does not show geometric or optical isomerism
Magnetic behaviour - DiamagneticIdentify the chiral molecule in the following pair:

A chiral molecule in which one of the carbon atom bearing four different groups. In the following pair of molecules, the chiral molecule is

(i) Write the IUPAC name of the complex [Cr(NH3)4Cl2]Cl.
(ii) What type of isomerism is exhibited by the complex [Co(en)3]3+?
(en = ethane-1,2-diamine)
(iii) Why is [NiCl4]2− paramagnetic but [Ni(CO)4] is diamagnetic?
(At. nos. : Cr = 24, Co = 27, Ni = 28)
(i) The IUPAC name of the complex [Cr(NH3)4Cl2]Cl is Tetraamminedichlorochromium(III) chloride.
(ii) The complex [Co(en)3]3+ exhibits optical isomerism. Its optical isomers are shown below.
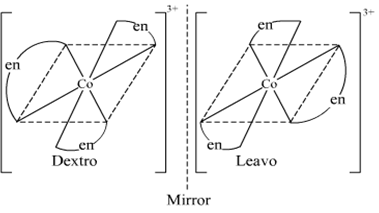
(iii) In [NiCl4]2−, the oxidation state of Ni is +2. Chloride is a weak field ligand and does not cause pairing up of electrons against the Hund's rule of maximum multiplicity. As a result, two unpaired electrons are present in the valence d-orbitals of Ni which impart paramagnetic character to the complex. On the other hand, carbonyl is a strong field ligand and causes pairing up of electrons against the Hund's rule of maximum multiplicity. As a result, no unpaired electrons are present and hence, the complex is diamagnetic.
What type of isomerism is shown by the complex [Co(NH3)6] [Cr(CN)6]?
[Co(NH3)6] [Cr(CN)6] are the examples of coordination isomerism. This isomerism occurs only in those complexes in which both cation and anion are complex. It occurs due to exchange of ligands between cation and anion.
Why a solution of [Ni(H2O)6]2+ is green while a solution of [Ni(CN)4]2– is colourless? (At. no. of Ni = 28)
In [Ni(H2O)6]+2 shows d-d transition t2g have 6 electrons and eg has 2 with octahedral structure and here d-d transition is allowed so it is coloured. While in [Ni(CN)4]2- the structure of the compound is square and hence has a centre of symmetry and hence it is Laporte forbidden and hence d-d transition is not allowed here with dsp2 hybridization.
Write the IUPAC name of the following complex : [Co(NH3)5(CO3)]Cl.
Pentaaminecarbonatocobalt(III)chloride
Write the coordination number and oxidation state of Platinum in the complex [Pt(en)2Cl2].
Coordination number and oxidation state of the given compound is given as,
Coordination no. = denticity × number of ligand
Coordination number = 2 × 2 + 2 × 1 = 6
Charge on ligand + O.S. of metal ion = charge on complex
– 2 + x = 0
⇒ x = +2
Write the formula of the following coordination compound :
Iron(III) hexacyanoferrate (II)
The formula of the given compound
Fe4[Fe(CN)6]3
Write the hybridisation and number of unpaired electrons in the complex [CoF6]3-.
Atomic No. of Co = 27
(i) Hybridization : sp3d2
(ii) Unpaired electron : 4-unpaired electron
Sc3+ is colourless in aqueous solution whereas Ti3+ is coloured.
Electronic configuration of Ti3+ is [Ar]3d2 4s2. Metal ions usually form hexacoordinated complexes with water molecules. According to Crystal field theory [Ti(H2O)6]+3, Ti3+ ion has outer shell configuration as There is an electron free to jump between energy level. Thus Ti3+ ions selectively absorb certain colours, hence its solution is coloured.
SC3+, on the other hand, has an empty d orbital; the [Sc(H2O)6]+3 complex has no electrons in T2g or eg orbitals. Hence SC3+ is colourless in aqueous solution.
Give reason:
When Cl2 reacts with an excess of F2. ClF3 is formed and not FCl3
Chlorine has empty d-orbital and it acquires excited state at the time of bonding when an electron from 3p-orbital are promoted to 3d- orbital.
In first excited state chlorine atom can exhibit a covalency of three, hence cannot expand its octet due to the absence of empty d- orbitals in 2nd energy shell.
Hence, it cannot exhibit covalency more than 1therefore FCl3 is not possible.
The pair having the same magnetic moment is:
[At. No. : Cr=24, Mn=25, Fe=26, Co=27]
-
[Cr(H2O)6 ]2+ and [Fe(H2O)6 ]2+
-
[Mn(H2O)6 ]2+ and [Cr(H2O)6 ]2+
-
[CoCl4 ]2− and [Fe(H2O)6 ]2+
-
[Cr(H2O)6 ]2+ and [CoCl4 ]2−
A.
[Cr(H2O)6 ]2+ and [Fe(H2O)6 ]2+
| Complex ion | Electronic configuration metal ion | Number of unpaired electrons (n) |
| [Cr(H2O)6 ]2+ | Cr2+ ; [ar] 3d4 | |
| [Fe(H2O)6 ]2+ | Fe2+ ; [Ar]3d6 | |
| [Mn(H2O)6 ]2+ | Mn2+ ; [Ar] 3d5 | |
| [CoCl4 ]2− | Co2+ ; [Ar]3d7 |
Which one of the following complexes shows optical isomerism?
-
cis[Co(en)2Cl2]Cl
-
trans[Co(en)2Cl2]Cl
-
[Co(NH3)4Cl2 ]Cl
-
[Co(NH3)3Cl3 ]
A.
cis[Co(en)2Cl2]Cl
The optically active compound capable of rotating the plane-polarized light to the right or left.
cis[Co(en)2Cl2]Cl is optically active compound.
The number of geometric isomers that can exist for square planar [Pt (Cl) (py) (NH3) (NH2OH)]+ is (py = pyridine)
-
2
-
3
-
4
-
6
B.
3
[Pt (Cl) (py) (NH3) (NH2OH)]+ is square planar complex.
The structures are formed by fixing a group and then arranging all the groups. Hence, this complex shows three geometrical isomers.
The Colour of KMnO4 is due to
-
M → L charge transfer transition
-
d-d transition
-
L →M charge transfer transition
-
σ →σ*
C.
L →M charge transfer transition
KMnO4 → K+ + MnO-4
Therefore,
In MnO4-, Mn has +7 oxidation state having no electron in d- orbitals.
It is considered that higher the oxidation state of metal, greater is the tendency to occur L →M charge transfer because ligand is able to donate the electrons into the vacant d- orbital of metal.
Since, charge transfer is Laporte as well as spin allowed, therefore, it shows colour.
Which of the following compounds will exhibit geometrical isomerism?
-
1-phenyl-2-butene
-
3-phenyl-1-butene
-
2-phenyl-1-butene
-
1,1-diphenyl-1-propane
A.
1-phenyl-2-butene
Alkene in which different groups are attached with the double bonded carbon atoms, exhibit geometrical isomerism.
The octahedral complex of a metal ion M3+ with four Monodentate Ligands, L1, L2, L3 and L4 absorb wavelengths in the region of red, green, yellow and blue respectively. The increasing order of ligand strength of the four ligands is
-
L4< L3<L2<L1
-
L1<L3<L2<L4
-
L3<L2<L4<L1
-
L1<L2<L4<L3
B.
L1<L3<L2<L4

| λ | L1 | L2 | L3 | L4 |
| Absorbed light | Red | Green | Yellow | Blue |
Therefore, increasing order of energy of wavelengths abosrbed reflects greater extent of crystal - field splitting, hence higher field strength of the ligand.
Energy: Blue(L4)> Green (L2)> Yellow (L3) > Red (L1)
Which of the following properties are not shown by NO?
-
It is diamagnetic in gaseous state
-
It is a neutral oxide
-
It combines with oxygen to form nitrogen dioxide
-
Its bond order 2.5
A.
It is diamagnetic in gaseous state
D.
Its bond order 2.5
NO is paramagnetic in the gaseous state because, in a gaseous state, It has one unpaired electron.
Total number of electron present = 7+8 = 15e-
Hence, there must be the presence of unpaired electron in a gaseous state while in a liquid state, it dimerises due to the unpaired electron.
Which of the following complex species is not expected to exhibit optical isomerism?
-
[Co(en)3]3+
-
[Co(en)2Cl2]+
-
[Co(NH3)3Cl3]
-
[Co(NH3)3Cl3]
C.
[Co(NH3)3Cl3]
Complexes of the type [MA2(AA2), [M(AA3)] exhibit optical isomerism.
Optical Isomerism is shown by only those complexes which lack elements of symmetry.
In the given complexes, [Co(NH3)3Cl3] shows facial as well as meridional isomerism. But both of the forms contain a plane of symmetry. Thus, only [Co(NH3)3Cl3] complex does not show optical isomerism.
Which one of the following molecules is expected to exhibit diamagnetic behaviour?
-
C2
-
O2
-
N2
-
S2
A.
C2
C2 and N2, all the electrons are paired, therefore, both of are diamagnetic.
C2 (6+6 = 12)
![]()
Oxygen and sulphur both are paramagnetic molecules.
Which one of the following statements is correct?
-
All amino acids except lysine are optically active
-
All amino acids are optically active
-
All amino acids except glycine are optically active
-
All amino acids except glutamic acid are optically active
C.
All amino acids except glycine are optically active
How many chiral compounds are possible on monochlorination of 2–methyl butane?
-
8
-
2
-
4
-
6
B.
2
In the above figure (II) and (IV) are chiral.
Which of the following facts about the complex [Cr(NH3)6]Cl3 is wrong?
-
The complex involves d2sp3 hybridisation and is octahedral in shape.
-
The complex is paramagnetic.
-
The complex is an outer orbital complex.
-
The complex gives a white precipitate with silver nitrate solution.
C.
The complex is an outer orbital complex.
In the case of d3 configuration, the number of unpaired electrons remains 3 whether the ligand is strong field or weak field. The hybridization scheme can be shown as follow :
Hence the complex is inner orbital complex as it involves (n - 1) d orbitals for hybridization, 3.93 = √n(n + 2) ; so n = 3 (where n is number of unpaired electron(s)).
The magnetic moment (spin only) of [NiCl4]2- is:
-
1.82 BM
-
5.46 BM
-
2.82 BM
-
1.41 BM
C.
2.82 BM
In the paramagnetic and tetrahedral complex [NiCl4]2-, the nickel is in +2 oxidation state and the ion has the electronic configuration 3d8. The hybridization scheme is as shown in the figure.
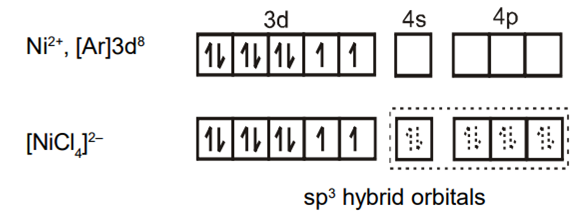
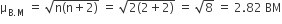
A solution containing 2.675 of CoCl3. 6NH3(molar mass = 267.5 g mol–1) is passed through a cation exchanger. The chloride ions obtained is solution was treated with excess of AgNO3 to give 4.78 g of AgCl (molar mass = 143.5 g mol–1 ). The formula of the complex is
(At. mass of Ag = 108 u)
-
[Co(NH3)6]Cl3
-
[CoCl2(NH3)4]Cl
-
[CoCl3(NH3)3]
-
[CoCl(NH3)5]Cl2
A.
[Co(NH3)6]Cl3
CoCl. 6NH3 + AgNO3 → AgCl
2.675/2667.5 excess 4.78/143.5
= 0.01 mole = 0.03310 mole
because 0.01 mole CoCl3.6NH3 given 0.0331 mole AgCl.
hence 1 mole of CoCl3.6NH3 will given 0.03310/0.010 =3 mole.
Thus the formula of the compound will be [Co(NH3)6]Cl3
Which one of the following has an optical isomer?
-
[Zn(en)(NH3)2]2+
-
[Co(H2O)4(en)]3+
-
[Co(en)3]3+
-
[Zn(en)2]2+
C.
[Co(en)3]3+
[Co(en)3]3+ is optically active and will give rise to optical isomers
Out of the following, the alkene that exhibits optical isomerism is
-
2–methyl–2–pentene
-
3–methyl–2–pentene
-
4–methyl–1–pentene
-
3–methyl–1–pentene
D.
3–methyl–1–pentene
3-methyl-1-pentene is optically active due to presence of chiral carbon, indicated by red star
The alkene that exhibits geometrical isomerism is
-
propene
-
2-methyl propene
-
2-butene
-
2- methyl -2- butene
C.
2-butene
2-butene may exist as 
Due to restricted rotation around double bond, it exhibits geometric isomerism.
Which of the following has an optical isomer ?
-
[CO(NH3)3Cl]+
-
[CO(en)(NH3)2]2+
-
[CO(H2O)(en)]3+
-
[CO(en)2(NH3)2]3+
D.
[CO(en)2(NH3)2]3+
It is an octahedral complex of the type[M(AA)2X2] where AA is a bidentate ligand.
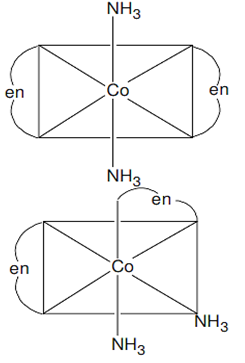
The number of stereoisomers possible for a compound of the molecular formula CH3-CH =CH-(OH)-Me is
-
3
-
2
-
4
-
6
C.
4
About the double bond, two geometrical isomers are possible and the compound is having one chiral carbon.
Which of the following pairs represents linkage isomers?
-
[Cu(NH3)4][PtCl4] and [Pt(NH3)4][CuCl4]
-
[Pd(PPh3)2(NCS)2] and [Pd(PPh3)2(SCN)2]
-
[CO(NH3)5NO3]SO4 and [CO(NH3)5SO4]NO3
-
[PtCl2(NH3)4]Br2 and [PtBr2(NH3)4]Cl2
B.
[Pd(PPh3)2(NCS)2] and [Pd(PPh3)2(SCN)2]
Linkage isomerism is shown by ambidient ligands like NCS and SCN. NCS- is ambidentate ligand and it can be linked through N (or) S
In which of the following octahedral complexes of Co (at. no. 27), will the magnitude of ∆o be the highest?
-
[Co(CN)6]3−
-
[Co(C2O4)3]3−
-
[Co(H2O)6]3+
-
[Co(NH3)6]3+
A.
[Co(CN)6]3−
CN- is stronger ligand hence ∆o is highest.
The absolute configuration of 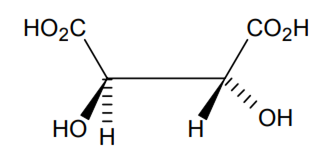
-
S,S
-
R,R
-
R,S
-
S,R
B.
R,R
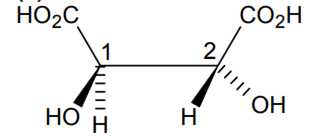
Both C1 and C2 have R – configuration.
Which one of the following has a square planar geometry?
-
[CoCl4]2-
-
[FeCl4]2–
-
[NiCl4] 2–
-
[PtCl4]2–
D.
[PtCl4]2–
27Co2+ - 1s2 2s2 2p6 3s2 3p6 3d7 4s0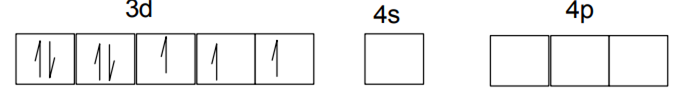
As Cl- is weak field ligand so no pairing up.Hence it is sp3 hybridized giving tetrahedral geometry.
Fe2+ - 1s2 2s2 2p6 3s2 3p6 3d6 4s0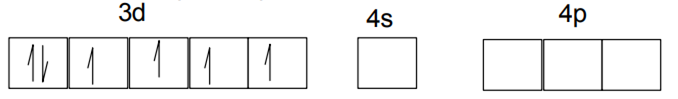
Due to Cl-, back pairing is not observed so it will be sp3 hybridized giving tetrahedral geometry.
Ni2+ - 1s2 2s2 2p6 3s2 3p6 3d8 4s0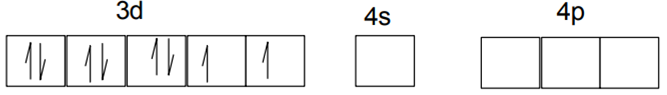
Because weak ligand, back pairing is not observed so it will be sp3 i.e. tetrahedral geometry.
All the complexes of Pt2+ are square planar including those with weak field ligand such as halide ions
In which of the following ionization processes, the bond order has increased and the magnetic behaviour has changed?
-
C2 → C2+
-
NO → NO+
-
O2 → O2+
-
N2 → N2+
B.
NO → NO+
D.
N2 → N2+
C2 → C2+ electron is removed from bonding molecular orbital so bond order decreases. In NO → NO+, electron is removed from antibonding molecular orbital so bond order increases and nature changes from paramagnetic to diamagnetic.
Which one of the following conformation of cyclohexane is chiral?
-
Twist boat
-
Rigid
-
chair
-
Boat
A.
Twist boat
The twisted boat is chiral as it does not have a plane of symmetry.
The IUPAC name for the complex [Co(NO2)(NH3)5]Cl2 is
-
nitrito-N-pentaamminecobalt (III) chloride
-
nitrito-N-pentaamminecobalt (II) chloride
-
pentaammine nitrito-N-cobalt (II) chloride
-
pentaammine nitrito-N-cobalt (III) chloride
D.
pentaammine nitrito-N-cobalt (III) chloride
The oxidation state of Cr in [Cr(NH3)4Cl2]+ is
-
+3
-
+1
-
0
-
+2
A.
+3
(Cr(NH3)4Cl2)+
X + 4×0 + 2×-1 = 1
X = +3
The value of the ‘spin only’ magnetic moment for one of the following configurations is 2.84 BM. The correct one is
-
d4 (in strong ligand filed)
-
d4 (in weak ligand field)
-
d3 (in weak as well as in strong fields)
-
d5 (in strong ligand field)
A.
d4 (in strong ligand filed)
d4 in strong field, so unpaired electrons = 2
For which of the following parameters the structural isomers C2H5OH and CH3OCH3 would be expected to have the same values?
-
Heat of vaporization
-
Gaseous densities at the same temperature and pressure
-
Boiling points
-
Vapour pressure at the same temperature
B.
Gaseous densities at the same temperature and pressure
Among the properties (a) reducing (b) oxidising (c) complexing, the set of properties shown by CN- ion towards metal species is
-
a, b
-
a, b, c
-
c, a
-
b,c
C.
c, a
CN- is a better complexing agent (C) as well as a reducing agent (A)
Thus properties (A) and (C) are shown
Property (C) Ni2+ + 4 CN- → [Ni(CN)4]2-
Property (A) CuCl2 + 5 KCN → K3[Cu(CN)4] + 1/2 (CN)2 + 2KCl
(CN- reduces Cu2+ to Cu+ )
The coordination number of central metal atom in a complex is determined by
-
The number of ligands around a metal ion bonded by sigma bonds
-
The number of only anionic ligands bonded to the metal ion
-
The number of ligands around a metal ion bonded by sigma and pi- bonds both
-
The number of ligands around a metal ion bonded by pi-bonds
A.
The number of ligands around a metal ion bonded by sigma bonds
Coordination number is the maximum covalency shown by a metal or metal ion. It is the maximum number of ligands attached to the metal by sigma bonds or coordinate bonds.
Coordination compound has great importance in biological systems. In this context which of the following statements is incorrect?
-
Chlorophylls are green pigments in plants and contain calcium
-
Carboxypeptidase – A is an enzyme and contains zinc
-
Cyanocobalamin is B12 and contains cobalt
-
Haemoglobin is the red pigment of blood and contains iron
A.
Chlorophylls are green pigments in plants and contain calcium
Chlorophyll contains Mg not Ca.
Which one the following has the largest number of isomers?
-
[Ru(NH3)4Cl2+]
-
[Co(en)2Cl2]+
-
[Ir(PR3)2H(CO)]2+
-
[Co(NH3)5Cl]2+
B.
[Co(en)2Cl2]+
[Co(en)2Cl2] forms optical and geometrical isomers.
The correct order of magnetic moments (spin only values in B.M.) among is
(Atomic numbers: Mn = 25; Fe = 26, Co =27)
-
[MnCl4]2- > [CoCl4]-2 > [Fe(CN)6]-4
-
[Fe(CN)6]-4 > [CoCl4]2- > [MnCl4]2-
-
[Fe(CN)6]4- > [MnCl4]2- > [CoCl4]2-
-
[MnCl4]2- > [Fe(CN)6]4- > [CoCl4]2
A.
[MnCl4]2- > [CoCl4]-2 > [Fe(CN)6]-4
Amongst the following compound, the optically active alkane having lowest molecular mass
D.
contains asymmetric carbon, thus optically active.
The trans-alkenes are formed by the reduction of alkynes with
Sn - HCl
H2 – Pd/C, BaSO4
NaBH4
Na/liq. NH3
D.
Na/liq. NH3
Na/Liq. NH3 reduces alkynes into trans alkene (trans addition).
Birch reduction is the anti addition. So trans alkene will be produced.

The oxidation states of Cr in [Cr(H2O)6]Cl3,[Cr(C6H6)2], and K2[Cr(CN)2 (O)2(O2)(NH3)] respectively are :
+3, +2, and +4
+3, 0, and +6
+3, 0, and +4
+3, +4, and +6
B.
+3, 0, and +6
(i) [Cr(H2O)6]Cl3:Hexaaquachromium(III) chloride
x + 6 × 0 + (–1) × 3 = 0
x = +3
(ii) [Cr(C6H6)2] : bis(h6–benzene)chromium(0)
y + 2 × 0 = 0
y =0 =
(iii) K2[Cr(CN)2 (O)2 (O2) (NH3)] :
Potassium amminedicyanidodioxidoperoxidochromate(VI)
2 × 1 + z + 2 × (–1) + 2 × (–2) + (–2) + 0 = 0
z = +6
The oxidation states of Cr in [Cr(H2O)6]Cl3, [Cr(C6H6)2], and K2 [Cr(CN)2 (O)2 (O2) (NH3)]
respectively are +3, 0 and +6.
Which of the following has longest C-O bond length? (free C-O bond length in CO is 1.128 A0)
-
[Co(CO)4]-
-
[Fe(CO)4]-2
-
[Mn(CO)6]+
-
Ni(CO)4
B.
[Fe(CO)4]-2
As the negative charge on metal carbonyl complex increases back pi bonding increases and hence the bond length of C-O bond increases while the bond length of metal-carbon bond decreases. Hence,[Fe(CO)4]2- has longest C-O bond length among the given complexes.
The correct order of bond length of the given complexes is
[Mn(CO)6]+<[Ni(CO)4]<[Co(CO)4]-<[Fe(CO)4]2-
Which of the following biphenyls is optically active?
A.
D.
The biphenyl compounds having a proper substitution at ortho-position of benzene rings result steric hindrance. This steric hindrance makes the biphenyl system non-planar and hence optically active compounds.
Which of these statements about [Co(CN)6]3- is true?
-
[Co(CN)6]3- has no unpaired electrons and will be in a low-spin configuration.
-
[Co(CN)6]3- has four unpaired electrons and will be in a low-spin configuration
-
[Co(CN)6]3- has four unpaired electrons and will be in high-spin configuration.
-
[Co(CN)6]3- has no unpaired electrons and will be in a high spin configuration.
A.
[Co(CN)6]3- has no unpaired electrons and will be in a low-spin configuration.
[Co(CN)6]-3
electronic configuration of Co : 1s2 2s2 2p6 3s2 3p6 3d6.
CN- is a strong field ligand and as it approaches the metal ion, the electrons must pair up.
The splitting of the d -orbitals into two sets of orbitals in an octahedral [Co(CN6)]3- may be represented as,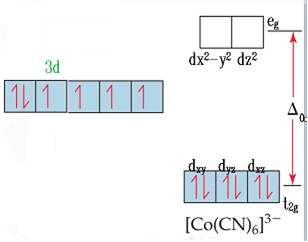
Here, for d6 ions, three electrons first enter orbitals with parallel spin put the remaining may pay up in t2g orbital giving rise to low spin complex.
Cobalt (III) chloride forms several octahedral complexes with ammonia, which of the following will not give a test for chloride ions with silver nitrated at 250 C?
-
CoCl. 3NH3
-
CoCl. 4NH3
-
CoCl. 5NH3
-
CoCl. 6NH3
A.
CoCl. 3NH3
a) [Co(NH3)3Cl3] --> [Co(NH3)2Cl3]
b) [Co(NH3)4Cl2]Cl --> [Co(NH3)4Cl2]+ +Cl-
c)[Co(NH3)5Cl]Cl2 --> [Co(NH3)5Cl]+ +2Cl-
d) [Co(NH3)6Cl]Cl2 --> [Co(NH3)6]+ +3Cl-
In the compound (a) does not ionise so does not give test for chloride ions.
Among the following complexes, the one which shows zero crystal field stabilisation energy (CFSE) is
-
[Mn(H2O)6]3+
-
[Fe(H2O)6]3+
-
[Co(H2O)6]3+
-
[Co(H2O)]3+
B.
[Fe(H2O)6]3+
The CFSE for octahedral complex is given by
CFSE = [-0.4 t2g e- +0.6 ege-]
For Mn3+, [3d4]--> t2g3e1g
therefore,
CFSE = [-0.4 x 3 + 0.6 x1]
For Fe3+, [3d] --> t2g3e2g
CFSE = [(-0.4 x 3) + (0.6 x2)] = 0
For Co2+, [3d7] --> t2g5e2g
CFSE = [(-0.4 x 5) + (0.6 x2)] =-0.8
For Co3+, [3d6] ---> t2g4e2g
CFSE = [(-0.4 x4) + (0.6 x2)] = -0.4
Which of the following complexes is used to be as an anticancer agent ?
-
mer=[Co(NH3)Cl3]
-
cis-[PtCl2(NH3)2]
-
cis-K2[PtCl2Br2]
-
Na2CoCl4
B.
cis-[PtCl2(NH3)2]
cis-platin is known as an anticancer agent. The formula for cis-platin is cis-[PtCl2(NH3)2]. Here, the word cis refers to a cis geometrical isomer of [PtCl2(NH3)2].
Which of the following compounds will undergo racemization when a solution of KOH hydrolysis?
-
(i) and (ii)
-
(iv)
-
(iii) and (iv)
-
(i) and(iv)
-
(i) and(iv)
B.
(iv)
Only compound (iv) result in the formation of racemic product due to chirality.other three do not show chirality.
Which of the following paramagnetic?
-
CO
-
O2-
-
CN-
-
NO+
B.
O2-
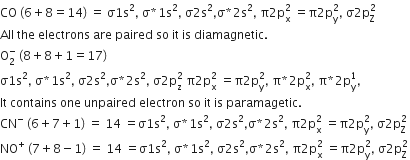
Thus, among the given species only O2- is paramagnetic.
An excess of AgNO3 is added to 100 mL of a 0.01 M solution of dichlorotetraaquachromium (III) chloride. The number of moles of AgCl precipitate would be
-
0.001
-
0.002
-
0.003
-
0.01
A.
0.001
The formula of dichlorotetraqua chromium (III) chloride is [Cr(H2O)Cl2]Cl.
On ionisation it generates only one Cl- ion.
[Cr(H2O)4Cl2]Cl --> [Cr(H2O)Cl2]+ +Cl-
Initial 100x0.01 0 0
0 1 mol 1 mol
One mole of Cl- ions reacts with only 1 mole of AgNO3 molecules to produce 1 mole of AgCl.
therefore, 1 mmol or 1 x 10-3 mole reacts with AgNO3 to give AgCl.
= 1 x 1 x 10-3/1 = 0.001 mol AgCl
Which of the following acids does not exhibit optical isomerism?
-
Maleic acid
-
α-amino acids
-
Lactic acid
-
Tartaric acid
A.
Maleic acid
Only those compounds exhibit optical isomerism, which has chiral centre and / Or absence of symmetry elements. (chiral carbon is the four valencies of which are satisfied by four different groups.)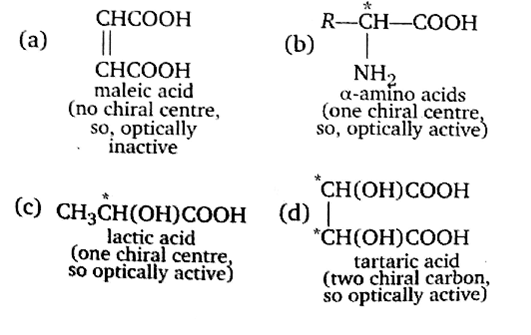
Thus, maleic acid does not exhibit optical isomerism.
Red precipitate is obtained when ethanol solution of dimethylglyoxime is added to ammoniacal Ni (II).Which of the following statements is not true?
-
Red Complex has a square planar geometry
-
Complex has symmetrical H- bonding
-
Red complex has a tetrahedral geometry
-
Dimethylglyoxime functions as bindentate ligand
C.
Red complex has a tetrahedral geometry
The reaction of Nickel with DMG Gives,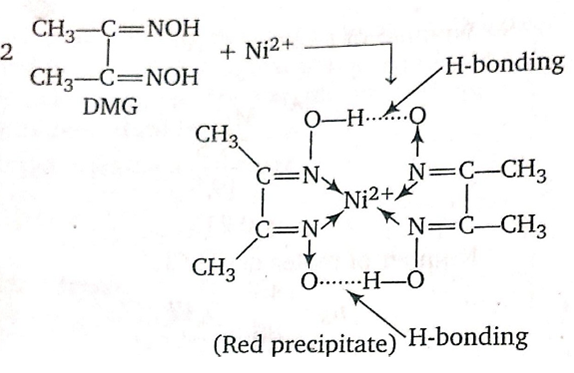
It shows that DMG acts as a bidentate ligand.
also, the geometry of DMG is square planar.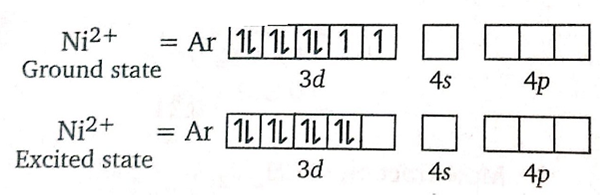
Hybridization of DMGi is dsp2 thus structure of is square planar.
Low spin complex of d6 -cation in an octahedral field will have the following energy
(Δo = Crystal field splitting energy in an octahedral field,
P= electron pairing energy)
A.
According to crystal field theory, splitting in octahedral field for low spin complex of d6-cation is shown as,
The complexes [Co(NH3)6][Cr(CN)6] and [Cr(NH3)6][Co(CN)6] are the examples of which type of which type of isomerism?
-
Ionisation isomerism
-
Coordination isomerism
-
Geometrical isomerism
-
Linkage isomerism
B.
Coordination isomerism
The complexes [Co(NH3)6] [Cr (CN)6] and [Cr(NH3)6][Co(CN)6] are the examples of coordination isomerism. This isomerism occurs only in those complexes in which both cation and anion are complex. It occurs due to exchange of ligands between cation and anion.
The d- electron configurations of Cr3+, Mn2+ , Fe2+, and Co2+ are d4 ,d5, d6 and d7 respectively. Which one of the following will exhibit minimum paramagnetic behaviour?
(At. no. Cr = 24, Mn = 25, Fe =26, Co = 27)
-
[Fe(H2O)6]2+
-
[Co(H2O)6]2+
-
[Cr(H2O)6]2+
-
[Mn(H2O)6]2+
B.
[Co(H2O)6]2+
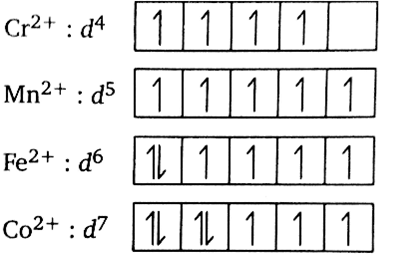
Therefore, [Co(H2O)6]2+ has a minimum number of unpaired electrons and thus, minimum paramagnetic behaviour.
Of the following complex ions, which is diamagnetic in nature?
-
[Ni(CN)4]2-
-
[CuCl4]2-
-
[CoF6]3-
-
[NiCl4]2-
A.
[Ni(CN)4]2-
Electronic configuration of Ni2+ in [Ni(CN)4]2- is Ni2+ = 3d8 4s0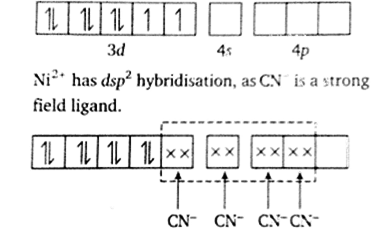
The complex, [Pt (Py)(NH3)BrCl] will have how many geometrical isomers?
-
4
-
0
-
2
-
3
D.
3
The complex is square planar and is of the type [M(abcd)]. It has three geometrical isomers.
The sum of coordination number and oxidation number of the metal M in the complex [M(en)2(C2O4)]Cl (where en is ethylenediamine) is
-
9
-
6
-
7
-
8
B.
6
Given complex compound is [M(en)2(C2O4)]Cl
Let oxidation number of M is x
therefore, x- 2 - 2 = -1
x = +3
Now, as coordination number is defined as the total number of binding sites attached to the metal.Hence, in the given complex coordination number is 6.
The name of complex ion, [Fe(CN)6]3- is
-
hexacyanoiron (III) ion
-
hexacyanitoferrate (III) ion
-
tricyanoferrate (III) ion
-
hexacyanidoferrate (III) ion
D.
hexacyanidoferrate (III) ion
When complex- ions is an anion, the name of the metal ends with the suffix -ate along with its oxidation number in the complex- ion.
[Fe(CN)6]3- = Hexacyanidoferrate (III) ion
Which of the following carbonyl will have the strongest C- O bond?
-
(Mn(CO)6+
-
Cr(CO)6
-
V (CO)6-
-
Fe(CO)5
A.
(Mn(CO)6+
As the positive charge on the central metal atom increase, the less readily the metal can donate electron density into the anti - bonding pi-orbitals of CO ligand to weaken the C-O bond. Hence, the C-O bond would be strongest in Mn(CO)6+.
The pair of species of oxygen and their magnetic behaviours are noted below. Which of the following presents the correct description?
C.
As O2+, O2, O2-, O and O+ have unpaired electrons, hence are paramagnetic. The species which have unpaired electrons has paramagnetic behaviour.
Which of the following complex compounds will exhibit highest paramagnetic behaviour ?
At. no. : Ti = 22, Cr = 24, Co = 27, Zn = 30
-
[Ti(NH3)6]3+
-
[Cr(NH3)6]3+
-
[Co(NH3)6]3+
-
[Zn(NH3)6]2+
B.
[Cr(NH3)6]3+
a) Electronic configuration of Ti3+ in [Ti(NH3)6]3+![]()
b) Electronic configuration of Cr3+ in [Cr(NH3)]3+
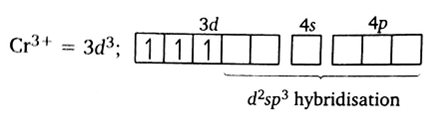
Electronic Configuration of Co3+ in [Co(NH3)6]3+
In the presence of strong field ligand NH3 pairing of electrons takes place and thus, octahedral complex, [Co(NH3)6]3+ is diamagnetic.
[Co(NH3)6]3+
Inner orbital or low spin complex
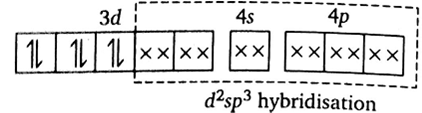
d) Electronic configuration of Zn2+ in [Zn(NH3)6]2+
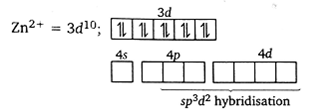
therefore, [Zn(NH3)6]2+ is an outer orbital complex and its diamagnetic .
Number of possible isomers for the complex [Co(en)2Cl2]Cl will be
(en =ethylenediamine)
-
2
-
1
-
3
-
4
C.
3
[Co(en)2Cl2]Cl
Possible isomers are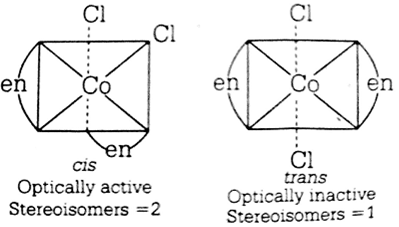
Hence,total number of stereoisomers = 2+1 = 3
The hybridization involved in complex [Ni(CN)4]2- is (Atomic number of Ni = 28)
-
dsp2
-
sp3
-
d2sp3
-
d2sp2
A.
dsp2
[Ni(CN)4]2-
Let oxidation state of Ni in [Ni(CN)4]2- is x.
x - 4 = - 2
Or
x= 2
Now, Ni2+ = [Ar] 3d8 4so![]()

therefore, hybridisation of [Ni(CN)4]2- is dsp2
Which of the following complex ions is not expected to absorb visible light?
-
[Ni(CN)4]2-
-
[Cr(NH3)6]3+
-
[Fe(H2O)6]2+
-
[Ni(H2O)6]2+
A.
[Ni(CN)4]2-
For the absorption of visible light, the presence of unpaired d- electrons is the necessity.
a) In [Ni(CN)4]2- , Ni is present as Ni2+
Ni2+ = [Ar] 3d8 4s0
Therefore, [Ni(CN)4]2- = 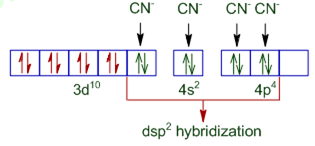
(pairing occurs because CN- is a strong field ligand).
Since in [NiCN)4]2-, no unpaired electron is present in d- orbitals, it does not absorb visible light.
(b) In [Cr(NH3)6]3+, [Fe(H2O)6]2+ [Ni(H2O)6]2+ has unpaired electrons such as
[Cr(NH3)6]3+ Cr3+ = Have three unpaired electron
[Fe(H2O)6]2+ Fe2+ = Have four unpaired electrons
[Ni(H2O)6]2+ Ni2+ = Have two unpaired electrons
Crystal field stabilisation energy for high spin d4 octahedral complex is
-
-1.8 Δo
-
-1.6 Δo + P
-
-1.2 Δo
-
-0.6 Δo
D.
-0.6 Δo
In the case of high spin complex Δo is small. Thus, the energy required to pair up the fourth electron with the electrons of lower energy d- orbitals would be higher than that required to place the electrons in the higher d -orbital. Thus pairing does not occur.
For high spin d4 octahedral complex,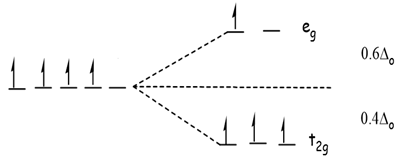
therefore,
Crystal field stabilisation energy
= (-3 x 0.4 +1 x 0.6) Δo
= - (-1.2 x 0.6 )Δo
=-0.6 Δo
The existence of two different coloured complexes with the composition of [Co(NH3)4Cl2]+ is due to
-
linkage isomerism
-
geometrical isomerism
-
Coordination isomerism
-
Ionisation isomerism
B.
geometrical isomerism
Complexes of [MA4B2] type exhibit geometrical isomerism.
The complex [Co(NH3)4Cl2]+ is a [MA4B2] type complex and thus, fulfils the conditions that are necessary to exhibit geometrical isomerism. Hence, it has two geometrical isomers
Which one of the following complexes is not expected to exhibit isomerism?
-
[Ni(NH3)(H2O)2]2+
-
[Pt(NH3)2Cl2]
-
[Ni(NH3)2Cl2]
-
[NI(en)3]2+
C.
[Ni(NH3)2Cl2]
[Ni(NH3)2Cl2] has tetrahedral geometry and thus, does not exhibit isomerism due to the presence of symmetry elements.
which of the following does not show optical isomerism? (en = ethylenediamine)
-
[Co(en)2Cl2]+
-
[Co(NH3)3Cl3]0
-
[Co(en)Cl2(NH3)2]+
-
[Co(en)3]3+
B.
[Co(NH3)3Cl3]0
optical isomerism is exhibited by only those complexes in which elements of symmetry are absent. Octahedral complexes of the types [M(aa)3], [M(aa)x2,y2] and [M(aa)2x2] have an absence of elements of symmetry, thus exhibit optical isomerism. Here, aa represents bidentate ligand, x or y represent monodentate ligand and M represent central metal ion.
Hence, [Co(NH3)3Cl3]o due to the presence of symmetry elements does not exhibit optical isomerism.
which of the following complex ions is expected to absorb visible light?
(At. no.Zn = 30, Sc = 21, Ti = 22, Cr = 24)
-
[Sc(H2O)(NH3)3]3+
-
[Ti(en)2(NH3)2]4+
-
[Cr(NH3)6]3+
-
[Zn(NH3)6]2+
D.
[Zn(NH3)6]2+
only those transition metal complexes are expected to absorb visible light, in which d-subshell is incomplete (ie, has unpaired electron) and excitation of an electron from a lower energy orbital to higher energy orbital is possible.
a) In [Sc(H2O)(NH3)3]3+ Sc is present in Sc3+ = [Ar]3d0,4s0
since in this complex excitation of the electron is not possible, it will not absorb visible light.
b) In [Ti(en)2(NH3)2]4+,Ti is present as Ti4+.
Ti4+ = [Ar] 3d0,4s0
Hence it will not absorb visible light.
c) In [Cr(NH3)6]3+ ,Cr is present as Cr3+
Cr3+ = [Ar]3d3,4s0
Since this complex has three unpaired electrons, excitation of electrons is possible and thus, it is expected that this complex will absorb visible light.
d) In [Zn(NH3)6]2+, Zn is present Zn2+
Zn2+ = [Ar] 3d10, 4s0
Hence, this complex will not absorb visible light.
Out of TiF62-, CoF63-, Cu2Cl2 and NiCl42- (Z of Ti = 22, Co= 27, Cu = 29, Ni =28) the colourless species are
-
TiF62- and CoF63-
-
Cu2Cl2 and NiCl42-
-
TiF62- and Cu2Cl2
-
CoF63- and NiCl42-
C.
TiF62- and Cu2Cl2
In Cu2Cl2, Cu is present as Cu+
electronic configuration of Cu+ [Ar] 3d10 4s0
thus, due to the absence of a unpaired electron, Cu2Cl2 is colourless.
In TiF62-, Ti present as Ti4+
Ti4+ = [Ar] 3d0 4s0
Hence, TiF62- is colourless.
Which of the following compound will exhibit cis-trans (geometrical) isomerism?
-
2-butene
-
Butanol
-
2-butyne
-
2-butenol
A.
2-butene
Compounds which have at least one double bond (C=C) and the groups attached with double bonded carbon atoms are different exhibit geometrical isomerism.
On the basis of the following Eo values; the strongest oxidising agent is
[Fe(CN)6]4- → [Fe(CN)6]3-] +e- ;
Eo = -0.35 V
Fe2+ → Fe3+ +e-; E = -0.77 V
-
[Fe(CN)6]4-
-
Fe2+
-
Fe3+
-
[Fe(CN)6]3-
C.
Fe3+
Oxidised form + ne- → Reduced Form
The substance which has lower reduction potential are stronger reducing agent while the substances which have higher reduction potential are a stronger oxidising agent.
[Fe(CN)6]3- + e- →[Fe(CN)6]4- ; Eo = 0.35 V
Fe3+ + e- → Fe2+ ; Eo = 0.77 V
The reduction potential of Fe3+/ Fe2+ is higher, hence, Fe3+ is a strongest oxidising agent.
Which of the following complexes exhibits the highest paramagnetic behaviour?
Where gly = glycine, en = ethylenediamine and bpy = bipyridyl moities
(At. no. ; Ti = 22, V = 23, Fe =26, Co = 27)
-
[V(gly)2 (OH)2 (NH3)2]+
-
[Fe(en)(bpy)(NH3)2]+
-
[CO(OX)2(OH)2]-
-
[Ti(NH3)6]3+
C.
[CO(OX)2(OH)2]-
Greater is the number of unpaired electrons, larger is the paramagnetism.
[V(gly)2(OH)2(NH3)2]+
V23 = [Ar] 4s2, 3d2
Oxidation state of V in [[V(gly)2(OH)2(NH3)2]+ is
x + (-1) x 2 + (-1) x 2 + (0) x 2 = +1
x=+5
V5+ = [Ar]3d0
[Fe(en)(bby)(NH3)2]2+
x + (0) + (0) + (0) x 2 = +2
x = +2
But en , bby and NH3 all are strong field ligands, so pairing occurs, thus no unpaired electrons.
[Co(OX)2 (OH)2]- is
Oxidation state of Co in [Co(OX)2(OH)2]- is
x + (-2) x 2 + (-1) x 2 = -1
x-6 = -1
x = +5
Co5+ = [Ar]3d4
[Ti(NH3)6]3+ is +3, thus it contains 1 unpaired electron.
Hence, [Co(OX)2(OH)2]- has highest paramagnetic behaviour.
How many stereoisomers does this molecule have?
![]()
-
4
-
6
-
8
-
2
A.
4
Number of optical isomers = 2n
where n = number of dissimilar asymmetric carbon atoms.
(ii) Number of geometrical isomers = 2n
Where n = a number of double bonds only when the two ends of the chain are different.
![]()
Here, number of optical isomers = 21 = 2
Number of geometrical isomers = 21 = 2
Hence, number of steroisomers = 2 + 2 = 4
In which of the following coordination entities the magnitude of Δo (CFSE in the octahedral field) will be maximum?
(Atomic number Co = 27)
-
[Co(H2O)6]3+
-
[CO(NH3)6]3+
-
[CO(CN)6]3-
-
[Co(C2O4)3]3-
C.
[CO(CN)6]3-
The magnitude of Δoct (the orbital splitting energy) is decided by the nature of ligand. Strong field ligand has highest Δoct .The increasing field strength is as
![]()
The CN- is the strongest ligand among these hence the magnitude of Δoct will be maximum in [Co(CN)6]3-
Which of the following will give a pair of enantiomorphs?
(en = NH2CH2CH2NH2)
-
[Co(NH3)Cl2]NO2
-
[Cr(NH3)6]Co(CN)6]
-
[Co(en)2Cl2]Cl
-
[Pt(NH3)][PtCl6]
C.
[Co(en)2Cl2]Cl
Enantiomorphs or Enantiomers: A Pair of molecules related to each other as an object to its mirror image are known as enantiomorphs or enantiomers. These are not superimposable on its mirror image.
The example is [Co(en)2Cl2]+
Dichlorobis [etylene diamine] cobalt (III)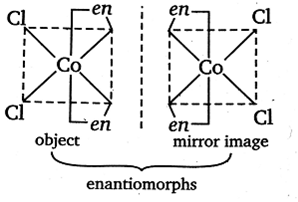
If there is no rotation of plane polarises light by a compound in a specific solvent, thought to by chiral, it may mean that:
-
the compound is certainly a chiral
-
the compound is certainly meso
-
there is no compound in the solvent
-
the compound may be a racemic mixture
D.
the compound may be a racemic mixture
An optically active compound rotate plane polarised light. But due to some external compensation (heat, light, catalyst, solvent etc,) the optically active compound loss their optical activity. The compound then exists as recemic mixture i.e, both d and l form are present in solution due to some intramolecular rearrangement.
So, there is no rotation of plane polarised light and compound does not have an asymmetric centre and when it again forms the symmetric centre bod d-and l forms are the obtained in equal amount.
CH3-CHCl-CH2-CH3 has a chiral centre. Which one of the following represents its R configuration?
A.
CH3 - CHCl - CH2- CH3
Priority order is
-Cl >-C2H5 > - CH3 > - H

The d-electron configuration of Cr3+, Mn2+, Fe2+ and Ni2+ are 3d4, 3d5, 3d6 and 3d8 respectively. Which one of the following aqua complexes will exhibit the minimum paramagnetic behaviour?
(At. no. Cr = 24, Mn = 25, Fe = 26, Ni = 28)
-
[Mn(H2O)6]2+
-
[Fe(H2O)6]2+
-
[Ni(H2O)6]2+
-
Cr(H2O)6]2+
A.
[Mn(H2O)6]2+
As the number of unpaired electron increases, the magnetic moment increases and hence the paramagnetic behaviour increases.
so, Cr2+ (22) = 3d4 - 4 unpaired electrons
Mn2+ (23) = 3d5 - 5 unpaired electrons
Fe2+ (24) = 3d6 - 4 unpaired electrons
Ni2+ (26) = 3d8 -2 unpaired electrons
So, [Ni(H2O)6]2+ exhibit minimum paramagnetic behaviour.
In which of the following pairs are both the ions coloured in aqueous solution?
(At. no.: Sc = 21, Ti = 22, Ni = 28, Cu = 29, Co = 2)
-
Ni2+, Ti3+
-
Sc3+, Ti3+
-
Sc3+,Co2+
-
Ni2+, Cu+
A.
Ni2+, Ti3+
Ni28 = 1s2s, 2s2, 2p6, 3s2,3p6,3d8,4s2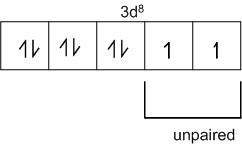
Ni2+ = 1s2, 2s2, 2p6, 3s2,3p6,3d8
Ti22 = 1s2,2s2, 2p6, 3s2,3p6,3d2,4s2
Sc21 = 1s2, 2s2, 2p6, 3s2,3p6,3d1,4s2
Sc3+ = 1s2, 2s2, 2p6, 3s2,3p6
(unpaired electron in d -orbital is not possible)
Cu29 = 1s2, 2s2, 2p6, 3s2,3p6,3d10,4s1
Cu+ = 1s2, 2s2, 2p6, 3s2,3p6,3d10
Hence, in above ions, Ni2+ and Ti3+ ions are coloured ions in aqueous solution due to presence of unpaired electrons in d -sub shell.
[Cr(H2O)6]Cl3 (at. no. of Cr = 24) has a magnetic moment of 3.83 BM, the correct distribution of 3d electrons in the chromium of the complex is:
C.
Magnetic moment,
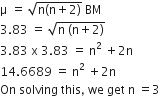
Hence, a number of unpaired electrons in d- subshell of the penultimate shell of chromium (Cr = 24).
so, the configuration of chromium ion is
Cr3+ = 1s2, 2s2, 2p6, 3s2, 3p6 3d3
In [Cr(H2O)6]Cl2 oxidation state of Cr is +3
Hence in 3d3 the distribution of electrons
![]()
An example of a sigma bonded organometallic compound is :
-
Ruthenocene
-
Grignard's reagent
-
Ferrocene
-
Cobaltocene
B.
Grignard's reagent
Grignard's reagent i.e., RMgX is σ-bonded organometallic compound.
The correct order of the stoichiometries of AgCl formed when AgNO3 in excess is treated with the complexes: CoCl3.6NH3, CoCl3.5NH3, CoCl3.4NH3 respectively is
-
1 AgCl, 3 AgCl, 2 AgCl
-
3 AgCl, 1 AgCl, 2 AgCl
-
3 AgCl, 2 AgCl, 1 AgCl
-
2 AgCl, 3 AgCl, 1 AgCl
C.
3 AgCl, 2 AgCl, 1 AgCl
Complexes are respectively [Co(NH3)6]Cl3, [Co(NH3)5Cl]Cl2and [Co(NH3)4Cl2]Cl
Correct increasing order for the wavelengths of absorption in the visible region for the complexes of Co3+ is
-
[Co(en)3]3+, [Co(NH3)6]3+, [Co(H2O)6]3+
-
[Co(H2O)6]3+, [Co(en)3]3+, [Co(NH3)6]3+
-
[Co(H2O)6]3+, [Co(NH3)6]3+, [Co(en)3]3+
-
[Co(NH3)6]3+, [Co(en)3]3+, [Co(H2O)6]3+
A.
[Co(en)3]3+, [Co(NH3)6]3+, [Co(H2O)6]3+
The order of the ligand in the spectrochemical series
H2O < NH3< en
Hence, the wavelength of the light observed will be in the order
[Co(H2O)6]3+, [Co(NH3)6]3+, [Co(en)3]3+
Thus, wavelength absorbed will be in the opposite order
[Co(en)3]3+, [Co(NH3)6]3+, [Co(H2O)6]3+
Match the metal ions given in Column I with the spin magnetic moments of the ions given in Column II and assign the correct code :
| Column I | Column II | ||
| a. | Co3+ | i. | |
| b. | Cr3+ | ii. | |
| c. | Fe3+ | iii. | |
| d. | Ni2+ | iv. | |
| v. | |||
a b c d iv v ii i a b c d i ii iii iv a b c d iii v i ii a b c d iv i ii iii
A.
| a | b | c | d |
| iv | v | ii | i |
Co3+ = [Ar] 3d6, Unpaired e–(n) = 4
Spin magnetic moment
Cr3+ = [Ar] 3d3, Unpaired e–(n) = 3
Spin magnetic moment =
Fe3+ = [Ar] 3d5, Unpaired e–(n) = 5
Spin magnetic moment =
Ni2+ = [Ar] 3d8, Unpaired e–(n) = 2
Spin magnetic moment =
Which one of the following ions exhibits d-d transition and paramagnetism as well?
C r O 4 2 - C r 2 O 7 2 - M n O 4 2 - M n O 4 -
C.
Iron carbonyl, Fe(CO)5 is
Tetranuclear
Mononuclear
Dinuclear
Trinuclear
B.
Mononuclear
Fe(CO)5
EAN = Z - O.N + 2 (C.N)
Here, Z = Atomic number
O.N = Oxidation number
C.N = Coordination number
= 26 - 0 + 2(5)
= 26 + 10
= 36
Only one central atom is present and it follows EAN rule, so it is mononuclear.
The type of isomerism shown by the complex [CoCl2(en)2] is
Geometrical isomerism
Coordination isomerism
Linkage isomerism
Ionization isomerism
A.
Geometrical isomerism
In the given complex, the CN of Co is 6, and the complex has octahedral geometry.

The geometry and magnetic behaviour of the complex [Ni(CO)4] are
Square planar geometry and diamagnetic
Tetrahedral geometry and diamagnetic
Tetrahedral geometry and paramagnetic
Square planar geometry and paramagnetic
B.
Tetrahedral geometry and diamagnetic
Ni(28) : [Ar]3d8 4s2
∵CO is a strong field ligand, so unpaired electrons get paired. Hence, the configuration would be.
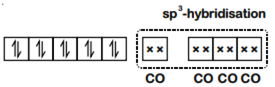
For, four ‘CO’-ligands hybridisation would be sp3 and thus the complex would be diamagnetic and of tetrahedral geometry.
Consider the following species:
CN+, CN–, NO and CN
Which one of these will have the highest bond order?
NO
CN-
CN
CN+
B.
CN-
Tips: -
Which of the following coordination compounds would exhibit optical isomerism?
Pentamminentirocobalt (III) iodide
Tris-(ethylenediamine) cobalt (III) bromide
Trans-dicyanobis (ethylenediamine)
Diamminedinitroplatinum (II)
B.
Tris-(ethylenediamine) cobalt (III) bromide
Tris-(ethylene diamine) Cobalt (III) bromide, i.e. [Co(en)3]Br3 exhibits optical isomerism.
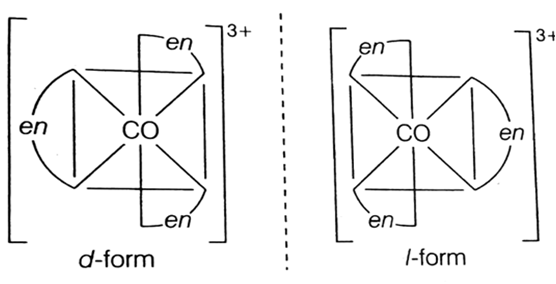
Sulphur reacts with chlorine in 1:2 ratio and forms X. Hydrolysis of X gives a sulphur compound Y. The hybridization of the central atom in the anion Y is
sp3
sp2
sp3d
sp
A.
sp3
when sulphur reacts with chlorine in 1:2 ratio the SCl4 is obtained which on hydrolysis gives H2SO4. Hence, the compound 'X' is SCl4 and 'Y' is H2SO4.
Mock Test Series
Sponsor Area
Sponsor Area