Sponsor Area
Coordination Compounds
What is meant by crystal field splitting energy? On the basis of crystal field theory, write the electronic configuration of d4 in terms of t2g and eg in an octahedral field when
(i) ![]() 0 > P
0 > P
(ii) ![]() 0 < P
0 < P
Crystal Field Splitting Energy: Crystal field theory was given to explain the structure and stability of the coordination complexes. This theory has some assumption like the metal ion is considered to be a point positive charge and the ligands are negative charge. The complexes are formed mainly by the d- block elements due to their variable oxidation states and variable coordination number. The d- subshell has 5 degenerate orbitals. when the ligand approach to the metal ion, the energy of the degenerate orbitals is increased and further splitting of degenerate orbital takes place into t2g and eg orbital. The difference between the t2g and eg orbitals is called as the crystal field splitting energy.
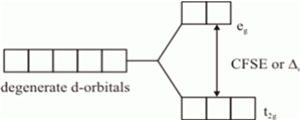
This splitting energy depends on the typo of ligand.
Strong field ligand will have high splitting energy and weak field ligand have low splitting energy.
For electric configuration of d4
(i) When 0 > P
In strong field ligand, the fourth electron will come back and pair in the t2g orbitals. So, the configuration will be Electronic configuration is

(ii) When o < P
In weak field ligand, the Electronic configuration is

Some More Questions From Coordination Compounds Chapter
Write the formula for the following coordination compound:
Dichloridobis(ethane-1, 2-diamine)platinum(lV) nitrate.
Write the formula for the following coordination compound:
Iron(III) hexacyanidoferrate(II)
Write the IUPAC names of the following coordination compounds:
(i) [Co(NH3)6]Cl3
(ii) [Co(NH3)5Cl]Cl2
(iii) K3[Fe(CN)6]
(iv) K3[Fe(C2O4)3]
(v) K2[PdCl4]
(vi) [Pt(NH3)2Cl(NH2CH3)]Cl.
Indicate the type of isomerism exhibited by the following complexes and draw structures for these isomers:
(i) K[Cr(H2O)2(C2O4)2], (ii) [Co(en)3Cl3,
(iii) [Co(NH3)5(NO2)]|NO3]2, (iv) [Pt(NH3)(H2O)Cl2]
(i) K[Cr(H2O)2(C2O4)2], (ii) [Co(en)3Cl3,
(iii) [Co(NH3)5(NO2)]|NO3]2, (iv) [Pt(NH3)(H2O)Cl2]
Explain on the basis of valence bond theory that [Ni(CN)4]2– ion with square planar is diamagnetic and the [NiCl4]2– ion with tetrahedral geometry is paramagnetic.
Predict the number of unpaired electrons in the square planar [Pt(CN)4,]2– ion.
Sponsor Area
Mock Test Series
Mock Test Series





