Write the hybridization and magnetic behaviour of the complex [Ni(CO)4].
(At.no. of Ni = 28)
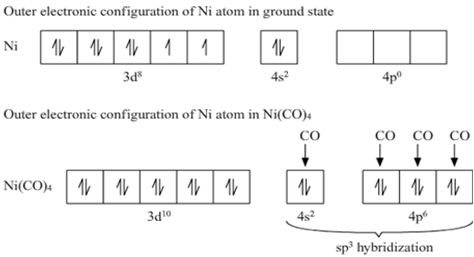
Carbonyl, CO being a strong field ligand causes the pairing of up valence electrons in the Ni atom against the Hund's Rule of Maximum Multiplicity. This results in the formation of an inner orbital complex, [Ni(CO)4] having diamagnetic character. [Ni(CO)4] has sp3 hybridization.



