Question
Which of the following paramagnetic?
-
CO
-
O2-
-
CN-
-
NO+
Solution
B.
O2-
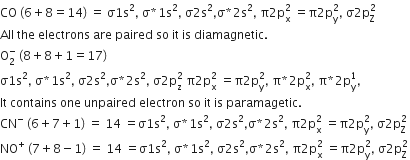
Thus, among the given species only O2- is paramagnetic.
Which of the following paramagnetic?
CO
O2-
CN-
NO+
B.
O2-
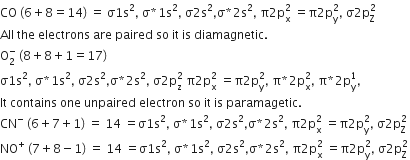
Indicate the type of isomerism exhibited by the following complexes and draw structures for these isomers:
(i) K[Cr(H2O)2(C2O4)2], (ii) [Co(en)3Cl3,
(iii) [Co(NH3)5(NO2)]|NO3]2, (iv) [Pt(NH3)(H2O)Cl2]
Predict the number of unpaired electrons in the square planar [Pt(CN)4,]2– ion.
Mock Test Series