Name of the following coordination entities and describe their structure:
(i) [Fe (CN)6]4-
(ii) [Cr (NH3)4Cl2]+
(iii) [Ni (CN) 4]2-
(Atomic numbers Fe = 26. Cr = 24, Ni = 28(i) [Fe (CN)6]4-
IUPAC name: Hexacyanoferrate (II)
Structure: Oxidation state of iron is + 2
Fe2+: Electronic configuration is 3d6 4s° 4p°
Orbitals of Fe2+ ions:
![]()
As CN- is a strong field ligand, it causes the pairing of unpaired 3d electrons
![]()
Since there are six ligands around the central metal ion, the most feasible hydrization is d2sp3.
d2sp2 hybridized orbitals of Fe2+ are:
![]()
6 electron pairs from CN-1 ions occupy the six hybrid d2sp3 orbitals
Then,

Hence, the structure of [Fe (CN)6]4- is octahedral.
(ii) [Cr (NH3)4Cl2] +
Name: Tetraamminedichlorido chromium (III)
Electronic configuration of Cr: 3d4 4s2
Electronic configuration of Cr3+: 3d3
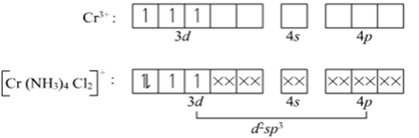
Structure is octahedral with d2sp3 hybridization.
(iii) [Ni (CN) 4]2-
Name: Tetracyanonickelate (II)
Structure: Here oxidation state of Ni is + 2
Ni + 2: Electronic configuration is 3d8 4s0 4p0
Orbitals of Ni2+ ion
![]()
As CN-1is a strong field ligand, it causes the pairing of unpaired 3d electrons.
![]()
Since, there are four ligands around the central metal ion, the most feasible hybridization is dsp2
![]()
4 electron pairs from CN-1 ions occupy the four hybrid dsp2 orbitals
Then,
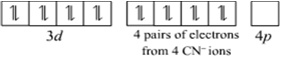
Hence, the structure of [Ni(CN)4]2- is square planar.





