The geometry and magnetic behaviour of the complex [Ni(CO)4] are
Square planar geometry and diamagnetic
Tetrahedral geometry and diamagnetic
Tetrahedral geometry and paramagnetic
Square planar geometry and paramagnetic
B.
Tetrahedral geometry and diamagnetic
Ni(28) : [Ar]3d8 4s2
∵CO is a strong field ligand, so unpaired electrons get paired. Hence, the configuration would be.
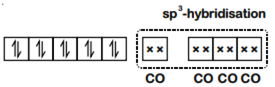
For, four ‘CO’-ligands hybridisation would be sp3 and thus the complex would be diamagnetic and of tetrahedral geometry.



