Red precipitate is obtained when ethanol solution of dimethylglyoxime is added to ammoniacal Ni (II).Which of the following statements is not true?
-
Red Complex has a square planar geometry
-
Complex has symmetrical H- bonding
-
Red complex has a tetrahedral geometry
-
Dimethylglyoxime functions as bindentate ligand
C.
Red complex has a tetrahedral geometry
The reaction of Nickel with DMG Gives,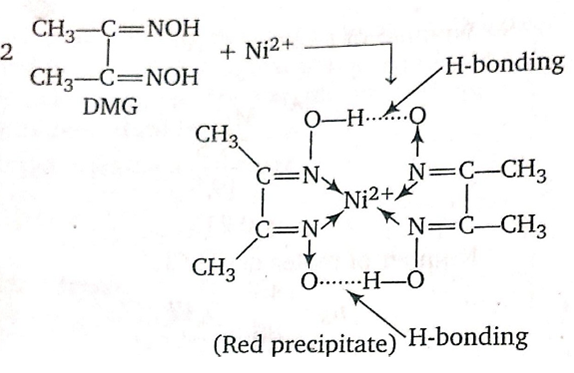
It shows that DMG acts as a bidentate ligand.
also, the geometry of DMG is square planar.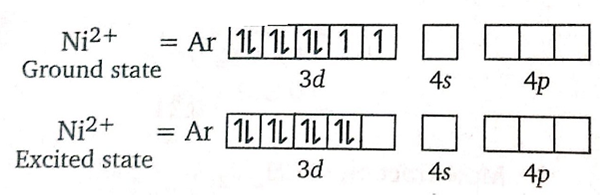
Hybridization of DMGi is dsp2 thus structure of is square planar.



