Sponsor Area
Coordination Compounds
The magnetic moment (spin only) of [NiCl4]2- is:
-
1.82 BM
-
5.46 BM
-
2.82 BM
-
1.41 BM
C.
2.82 BM
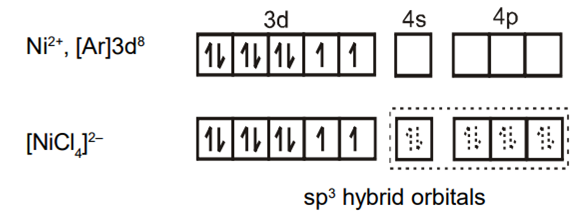
In the paramagnetic and tetrahedral complex [NiCl4]2-, the nickel is in +2 oxidation state and the ion has the electronic configuration 3d8. The hybridization scheme is as shown in the figure.
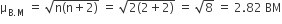
Some More Questions From Coordination Compounds Chapter
Write the formula for the following coordination compound:
Iron(III) hexacyanidoferrate(II)
Write the IUPAC names of the following coordination compounds:
(i) [Co(NH3)6]Cl3
(ii) [Co(NH3)5Cl]Cl2
(iii) K3[Fe(CN)6]
(iv) K3[Fe(C2O4)3]
(v) K2[PdCl4]
(vi) [Pt(NH3)2Cl(NH2CH3)]Cl.
Indicate the type of isomerism exhibited by the following complexes and draw structures for these isomers:
(i) K[Cr(H2O)2(C2O4)2], (ii) [Co(en)3Cl3,
(iii) [Co(NH3)5(NO2)]|NO3]2, (iv) [Pt(NH3)(H2O)Cl2]
(i) K[Cr(H2O)2(C2O4)2], (ii) [Co(en)3Cl3,
(iii) [Co(NH3)5(NO2)]|NO3]2, (iv) [Pt(NH3)(H2O)Cl2]
Explain on the basis of valence bond theory that [Ni(CN)4]2– ion with square planar is diamagnetic and the [NiCl4]2– ion with tetrahedral geometry is paramagnetic.
Predict the number of unpaired electrons in the square planar [Pt(CN)4,]2– ion.
The hexaquo manganese(II) ion contains five unpaired electrons, while the hexacynoion contains only one unpaired electron. Explain using crystal field theory.
Sponsor Area
Mock Test Series
Mock Test Series





