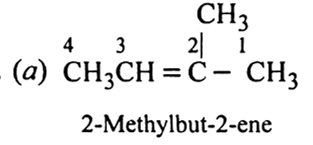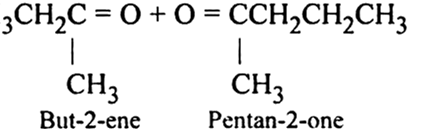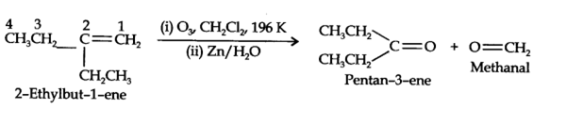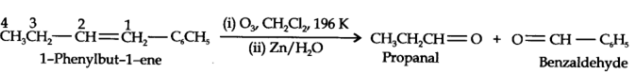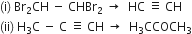Chemistry Part Ii Chapter 13 Hydrocarbons
Sponsor Area
NCERT Solution For Class 11 Business%2bstudies Chemistry Part Ii
Name the products formed when an ethereal solution containing ethyl iodide and methyl iodide is heated with sodium metal.
Alkanes are less reactive. Why?
During the pyrolysis of alkanes C-C bonds break in preference to C-H bond. Why?
The bond dissociation energy of C-C bond is less than that of C-H bond. Hence, C-C bonds break in preference to C-H bond.
Define cracking.
The thermal decomposition of higher hydrocarbons into lower hydrocarbons in the presence or absence of a catalyst is called cracking.
What is the function of iodic acid in the iodination of alkanes? Can we use any other substance in its place?
What is alternation effect?
n-alkanes with an even number of carbon atoms have much higher melting points than the next lower n-alkane with an odd number of carbon atoms.
Arrange the following compounds in order of their increasing boiling points:
n-Pentane, n-Hexane, Ethane, 2-2-Dimethylpentane, 2-Methylpentane.
Ethane < Pentane < 2-Methylpentane < n-Hexane < 2, 2-Dimethylpentane.
Why is light or heat necessary to initiate chlorination reaction of alkanes?
The Cl—Cl bond must be broken to form chlorine free radicals before the reaction with an alkane can commence. Thus, homolysis needs energy which is supplied either by light or heat.
What happens when steam is passed through methane at 1273 K in the presence of nickel catalyst?
Methane reacts with steam at 1273 K in the presence of nickel catalyst to form carbon monoxide and dihydrogen.
How is iodination of alkane shifted in the forward direction?
Iodination is extremely slow and reversible in nature.
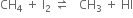
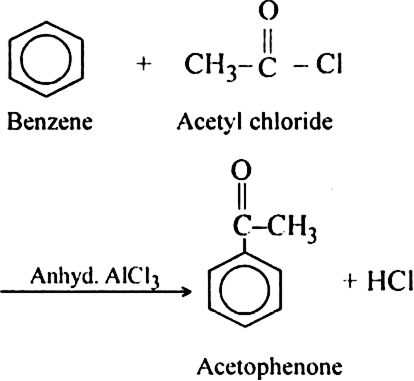
HI is a strong reducing agent and converts methyl iodide back to methane. In order to carry out the reaction in the forward direction. HI is destroyed with the help of an oxidising agent like iodic acid (HIO3), concentrated. HNO3 or mercuric oxide (HgO).
What are conformation or rotational isomers?
The infinite number of momentary arrangement of the atoms in space which result through rotation about a single or a band are called conformations or rotational isomers.
Out of eclipsed and staggered conformation of ethane, which is more stable?
Can you separate the two conformations of ethane?
Why are alkenes called olefins?
Alkenes are knowns as olefins (oil forming) since the first member, ethylene or ethene was found to form an oily liquid on reaction with chlorine.
Sponsor Area
How is the position of the double bond in an alkene located?
The position of the double bond in an alkene located by ozonolysis.
What is Lindlar's catalyst? What for is it used?
Which compound on polymerisation gives Teflon?
What is teflon? What for is it used?
Poly (tetrafluoroethylene) is called teflon. It is used for making non-stick utensils.
How will you detect the presence of unsaturation in an organic compound?
The presence of unsaturation in an organic compound can be detected by Baeyer's reagent or by Br2 in CCl4.
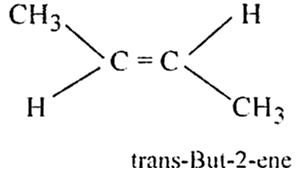
What is cis-trans isomerism?
The stereoisomerism arising due to restricted rotation of bonds is called cis-trans isomerism. The isomer which has similar groups on the same side of the double bond is called cis isomer and the isomer which has same groups on the opposite side of the double bond and is known as trans form.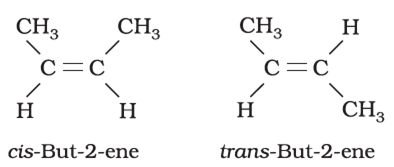
Which one of the following do not exhibit geometrical isomerism?
(i) Pentene-1 (ii) Butene-2
(iii) Isobutylene (iv) 3, 4-Dimethyl- 3-hexene.
What happens when water is dropped over calcium carbide?
Acetylene is formed.
CaC2(s) +2H2O(l) --> C2H2 (g)+Ca(OH2)(g)
Acetylene has garlic odour. Why?
This is because of the presence of impurities of phosphine and hydrogen sulphide.
What happens when propyne is oxidised with Baeyer's reagent (alkaline KMnO4) at 298 - 303 K?
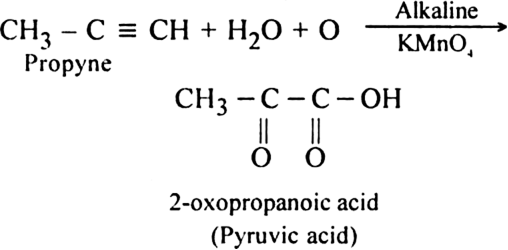
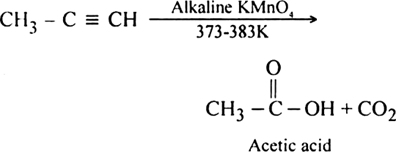
What happens when propyne is oxidised with alkaline KMnO4 at 373-383 K?
What happens when acetylene is treated with ammoniacal silver nitrate solution?
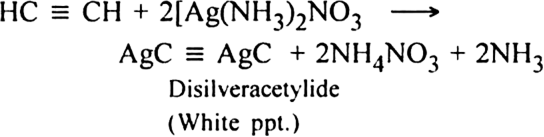
Mention two reagents which can be used to distinguish between ethene and ethyne.
(i) Ammoniacal silver nitrate (Tollen's reagent).
(ii) Ammoniacal cuprous chloride solution.
Although acetylene is acidic in nature, yet it does not react with NaOH or KOH. Explain.

List the following in order of increasing acidity: propyne, water, propene and propane.
The order of increasing acidity is,
propane < propene < propyne < water.
What are benzenoids?
Benzenoids are aromatic compounds which contain one or more benzene rings in their molecules. For
example benzene, napthalene etc.
Sponsor Area
What are non-benzenoids?
These are cyclic compounds which do not contain a benzene ring in their molecules. For example heterocyclic compounds and aromatic ions.
How will you represent a benzene molecule?
Benzene is generally represented as a regular hexagon containing a dotted or solid line circle.
Carbon is present at each corner of the hexagon and a hydrogen is attached to each carbon atom. The circle denotes the cloud of 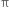 -electrons.
-electrons.
How many isomeric disubstitution derivatives are possible for benzene molecule?
Benzene can form three isomeric disubstitution derivatives known as ortho, meta and para according to as the substituents attached to adjacent or diagonal carbon atoms respectively. 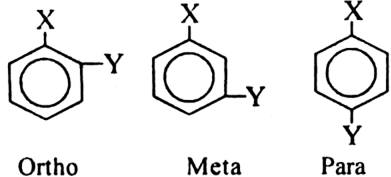
Write the formulae and names of all possible isomers of arenes having the molecular formula C8H10.
The possible arenes having the molecular formula C8H10 are: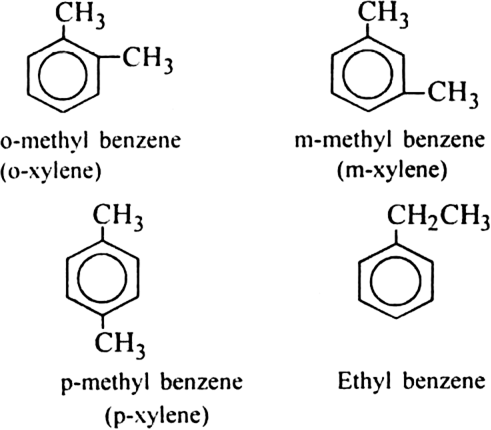
What is the state of hybridization of carbon atoms in:![]()
(iii) – C ≡ C –
(iv) C – C bond in benzene ?
(i) sp3 (ii) sp2 (iii) sp (iv) sp2.
What does the dotted circle inside the benzene ring indicate?
A delocalised ring of 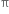 -electrons
-electrons
Why are all C - C bond lengths in benzene equal?
Due to the resonance all C-C bond lengths in benzene equal.
What is delocalisation?
Delocalisation can be defined as electron belonging to certain molecules are not attached to a particular atom or bond in that molecule. For example, benzene is a delocised molecule.
What is delocalisation energy?
A compound with delocalized electrons is more stable than it would be if all of its electrons were localised. The extra stability a compound gains from having delocalized electrons are called delocalization energy or resonance energy.
What is aromaticity?
Aromaticity is a property of conjugated cycloalkenes in which the stabilisation of the molecule is enhanced due to the ability of the electrons in the π orbitals to delocalize.
State Huckel's rule.
It states that a compound is said to be aromatic if it contains 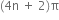 electrons, where n is a whole number of ring compounds. For example; cyclopropenyl cation.
electrons, where n is a whole number of ring compounds. For example; cyclopropenyl cation.
Here n = 0; 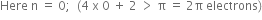
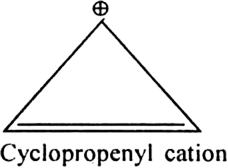
Name three heterocyclic compounds which are aromatic in nature.
Furan, thiophene and pyrrole.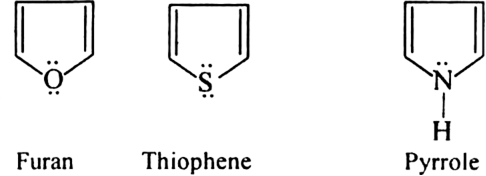
Each contains  electrons which satisfy Huckel's rule when n = 1.
electrons which satisfy Huckel's rule when n = 1.
Will cyclo-octatetraene show aromatic character?
 -electrons i.e. does not obey Huckel rule. It is non-aromatic.
-electrons i.e. does not obey Huckel rule. It is non-aromatic.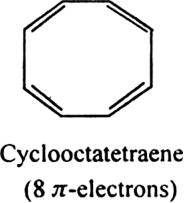
Cyclobutadiene is not aromatic. Explain.
Cyclobutadiene (C4H4) is not aromatic because:
(i) It is not coplanar. It is envelope shaped.
(ii) It has only four  -electrons and does not fulfil Huckel’s rule.
-electrons and does not fulfil Huckel’s rule.
what are annulenes? Give one example.
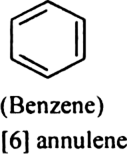
[6] Annulene is aromatic because it contains
 .
.Which of the following exhibits aromaticity:
(i) Cyclo - octatetraene (ii) Cyclopentadiene (iii) Tropylium cation?
 ] electrons.
] electrons.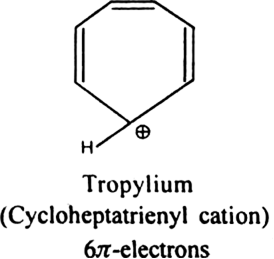
Give two examples of ions which are non-aromatic. Why don't they exhibit aromaticity?
 electron rule.
electron rule.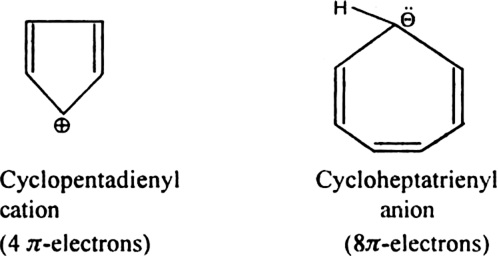
Is benzene same as 1, 3, 5-cyclohexatriene?
No benzene is not same as 1,3,5,-cyclohexatriene.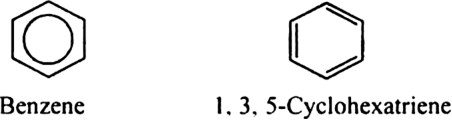
In 1, 3, 5-cyclohexatriene, there are three C = C having a bond length 134 pm and three C - C having a bond length 154 pm. In benzene, all the six C-C bonds have the same bond length 139 pm. Thus, benzene is not same as 1, 3, 5-cyclohexatriene.
What is the role of catalyst in electrophilic substitution reactions?
The catalyst helps to generate an electrophile from the attacking reagent. For example, ferric chloride (electron deficient) is used as a catalyst in the chlorination of benzene. It reacts with attacking chlorine molecule and forms chloronium ion (Cl+).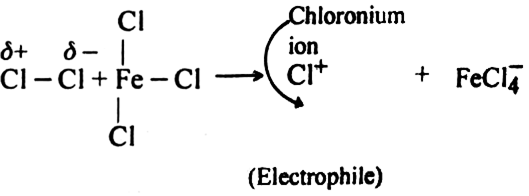
Name the catalysts used in electrophilic aromatic substitution reactions.
It may be either Lewis acids (such as anhydrous AlCl3 or ferric chloride) or protonic acids (such as H2SO4).
Suggest the name of a Lewis acid other than anhydrous aluminium chloride which can be used during ethylation of benzene.
Classify the following groups as activating or deactivating with respect to further electrophilic substitution of aromatic ring:
(a) – Cl (b) -CN
(c) – NH2 (d) –SO3H
(e) –NO2 (f) – CH3.
Define Wurtz Reaction?
Alkyl halides on treatment with sodium metal in dry ethereal solution give higher alkanes. This reaction is known as Wurtz reaction and is used for the preparation of higher alkanes containing an even number of carbon atoms.
What is lindane?
Benzene hexachloride (BHC). It is used both as an agricultural insecticide and as a pharmaceutical treatment for lice and scabies.
Last traces of water can be removed from benzene by using sodium metal. Why?
Last traces of water can be removed from benzene by using sodium metal because benzene does not react with sodium metal while water does. Hence, water is eliminated by reaction with sodium.
What is paraffinic petroleum and asphaltic petroleum?
Paraffinic petroleum contains a large proportion of alkanes while asphaltic petroleum contains a large proportion of cycloalkanes.
What is solvent naphtha? What is its true?
During coal-tar distillation, various fractions can be taken. The fraction distilling between 413-433 K is called solvent naphtha. It mainly contains xylenes and cumene and is used as a solvent for resins, rubbers, paints, etc.
What is knocking?
The metallic sound (knocking sound) produced due to irregular burning of fuel is known as knocking.
What is the octane number of:
(i) n-heptane (ii) iso-octane?
(i) n-heptane: zero
(ii) Iso-octane : 100
A sample of gasoline produces the same knocking as a mixture containing 35% n-heptane and 65% iso-octane. What is octane rating of the sample?
The octane rating of the given sample is 65. Since n-heptane has zero octane number.
Out of 2, 2, 3-trimethylbutane, 2,2 4-trimethylpentane and 2, 2, 3, 3,-tetramethyl butane which has the highest octane number? Explain.
The octane number increases as the branching increases, therefore, 2, 2, 3, 3-tetramethyl butane having four branches has the highest octane number.
Which compound of lead is added to gasoline (petrol) to increase its octane number?
Tetraethyl lead.
What is the cause of lead pollution of air?
Tetraethyl lead added to petrol as an antiknock compound.
Arrange the following in order of increasing volatility: gasoline, kerosene and diesel.
Increasing volatile order:
Diesel< kerosene< gasoline.
What is gasoline? What is its use?
Gasoline is a complex liquid mixture of hydrocarbons. It is used for making petrol.
Sponsor Area
What are petrochemicals?
All those chemical compounds which are derived directly or indirectly from petroleum or natural gas are called petrochemicals.
Why is petroleum called liquid gold?
Petroleum is called liquid gold because it has been found to be more precious than gold. Petroleum serves as a source for so many organic compounds which are highly useful to mankind directly or indirectly. It also helps in the economy of the country.
What are the main constituents of LPG?
What are alkanes and cycloalkanes ?
| n = 1 | CH4 | Methane |
| n = 2 | C2H6 | Ethane |
| n = 3 | C3H8 | Propane |
| n = 4 | C4H10 |
Butane |
| n = 5 | C5H12 | Pentane |
| n = 6 | C6H14 | Hexane |
| n = 7 | C7H16 | Heptane |
| n = 8 | C8H18 | Octane |
| n = 9 | C9H20 | Nonane |
| n = 0 | C10H22 | Decane |
Cycloalkanes: Saturated alicyclic hydrocarbons in which all the carbon atoms are joined by single covalent bonds are called cycloalkanes. These are represented by the formula CnH2n if they are monocyclic and CnH2n_2 in case they are bicyclic. For example,
Discuss the structures of alkanes.
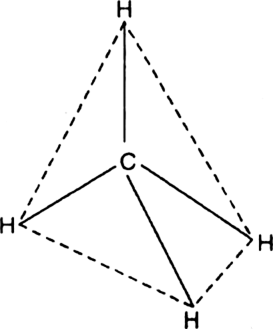
Therefore, carbon in alkanes is sp3 - hybridised. Since sp3 hybridised carbon has four half filled sp3 - orbitals, therefore, it forms four σ-bonds. These four bonds are directed towards the corners of a regular tetrahedron. In other words, the angle between any two adjacent bonds is 109° - 28' (tetrahedral angle).
In methane molecule, carbon lies at the centre of the tetrahedron while the four hydrogens are present at the corners of vertices of the regular tetrahedron. This implies that; each face of the tetrahedron is an equilateral triangle and has three bonds.
In the alkane H3C - CH2 -C(CH3)2 -CH2 - CH(CH3)2, identify 1°, 2°, 3° carbon atoms and give the H atoms bounded to each one of these.
Write the structure of the given compound in expanded form.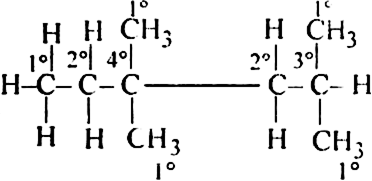
15H attached to five 1° carbons
4H attached to two 2° carbons
1H attached to one 3° carbon
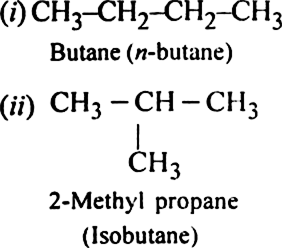
Write structures of different chain isomers of butane (C4H10).
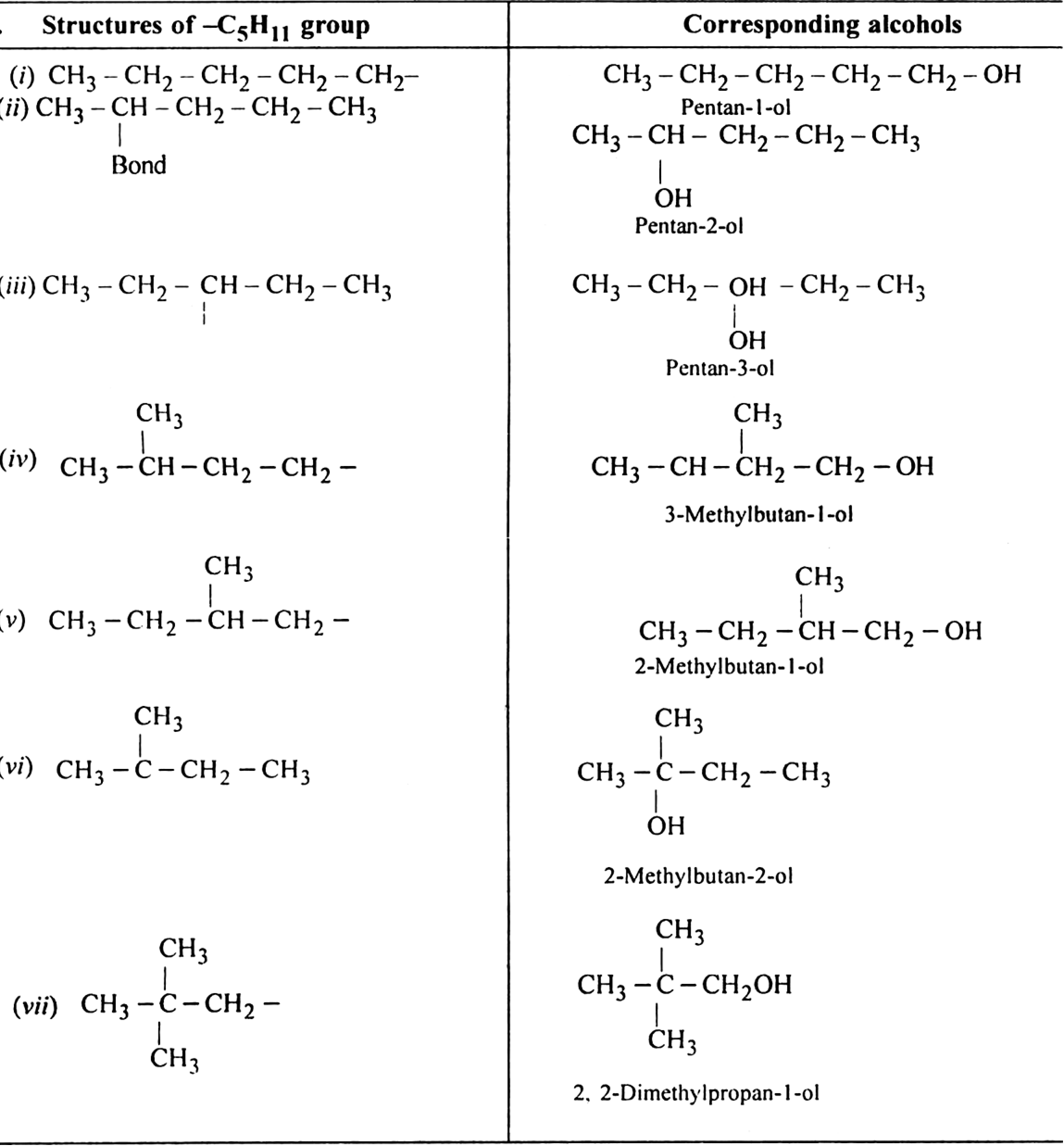
In how many ways, you can join five carbon atoms and twelve hydrogen atoms of C5H12 ?
They can be arranged in three ways: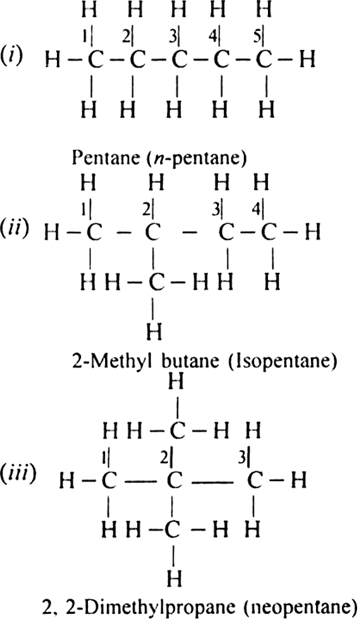
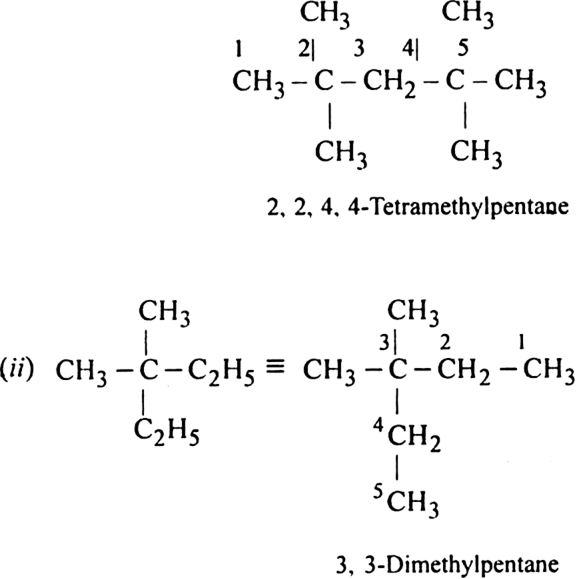
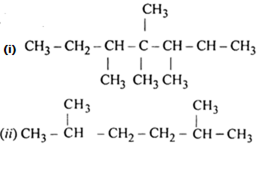
Write structures of different chain isomers of alkanes corresponding to the molecular formula C6H14. Also, write their IUPAC names.
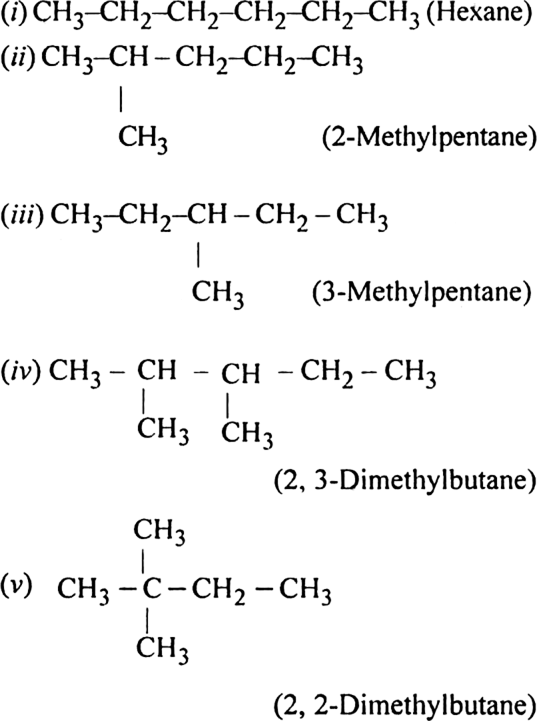
Write structures for each of the following compounds. Why are the given names incorrect? Write correct IUPAC names:
(i) 2-Ethylpentane
(ii) 5-Ethyl-3-methylheptane
Largest chain is of six carbon atoms and not that of five:
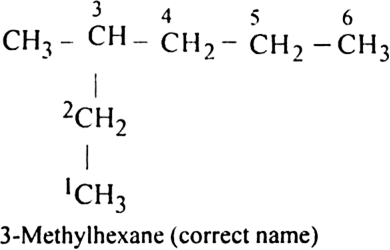
(ii) Structure of 5-Ethyl-3-methylheptane is:
Numbering is to be started from the end which gives the lowest number to ethyl group

How are alkanes formed from:
(i) unsaturated hydrocarbons
(ii) sodium salt of fatty acids?
(i) From unsaturated hydrocarbons: It consist of passing hydrogen gas through alkenes or alkynes in the presence of finely divided nickel at 523 - 573 K. Saturated hydrocarbons are produced.
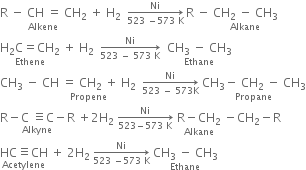
These are known as Sabatier and Senderen's reactions.
Methane can not be prepared by this method.
(ii) From sodium salt of fatty acids. The sodium salt of carboxylic acids on strong heating with soda lime (NaOH + CaO) yield alkanes. The reaction is termed as decarboxylation reaction since it involves the removal of carbon dioxide.


How are alkanes formed from alkyl halides?
Preparation from alkyl halides. Alkanes can be prepared from alkyl halides by the following methods:
1. By Wurtz’s reaction. It involves the chemical reaction between an alkyl halide and metallic sodium in the presence of dry ether. Two molecules of alkyl halide will react to form higher alkanes.


Methane cannot be prepared by this method.
When we use mixed alkyl halides, for example, methyl bromide and ethyl bromide, more than one product are obtained. 


This reaction is not suitable to prepare pure alkane with an odd number of carbon atoms because of side reactions.
Mechanism of Wurtz’s reaction: Two different mechanisms have been proposed.
(a) Intermediate formation of an organometallic compound:
(b) Intermediate formation of free radical: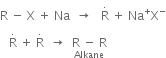
2. By Corey-House reaction: By Corey-House reaction: An alkyl halide is first treated with lithium metal in dry ether to form alkyl lithium. This then reacts with cuprous iodide to yield lithium dialkyl copper and which on subsequent treatment with a suitable alkyl halide gives the desired alkane.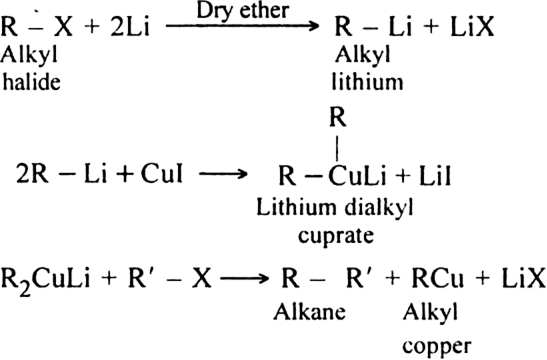
(R and R' may be same or different alkyl groups)
For example:
This reaction is very useful for the preparation of both symmetrical and unsymmetrical alkanes.
3. By the reduction of alkyl halides: The reduction of alkyl halide is carried:
(i) With zinc-copper and alcohol or zinc and HCl or zinc and NaOH: Zinc reacts with alcohol to produce nascent hydrogen which brings about reduction.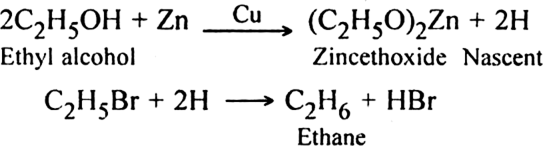
(ii) By catalytic reduction: By using hydrogen gas in the presence of platinum or palladium.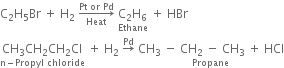
(iii) With red phosphorus and hydrogen iodide: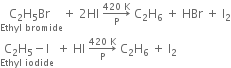
The function of red phosphorus is to combine with iodine to form phosphorus tri-iodide PI3. If it is not done, iodine will react back with an alkane to form alkyl halide.
4. Through the formation of Grignard reagent: Alkyl halides react with dry magnesium metal in the presence of anhydrous ether to form alkyl magnesium halides, also called Grignard reagent.
Grignard reagent can be easily decomposed by water or alcohol to form alkane.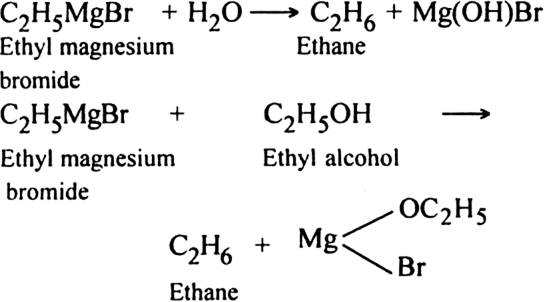
Why is Wurtz reaction not preferred for the preparation of alkanes containing odd number of carbon atoms? Illustrate your answer bytaking one example.
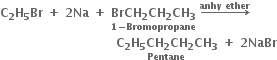
Butane product will also be formed when the numbers participating in the reaction react separately. For example, bromoethane will give butane and 1-bromopropane will give rise to hexane.
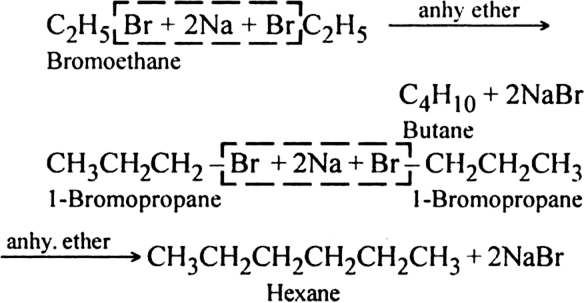
Thus, a mixture of butane, pentane and hexane will be formed. It will be quite difficult to separate the individual components from the mixture.
Give a brief account of Kolbe's electrolysis.
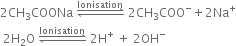
On passing electricity, the ions will move towards the respective electrodes.
At anode: The electron releasing tendency of CH3COO- ions is more and these are discharged in preference to OH- ions.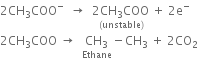
At cathode: The electron accepting tendency of H+ ions is more and these are discharged in preference to Na+ ions which remain in solution.

Limitations:
(i) Only alkanes with an even number of carbon atoms can be formed.
(ii) Methane cannot be prepared by this method.
Discuss the laboratory preparation of methane.

Experiment: Powdered sodium acetate is mixed with four times the amount of soda lime. The mixture is taken in a hard glass tube. It is fitted with the delivery tube.
As the contents of the tube are heated, methane gas is produced. It is collected by the downward displacement of water.
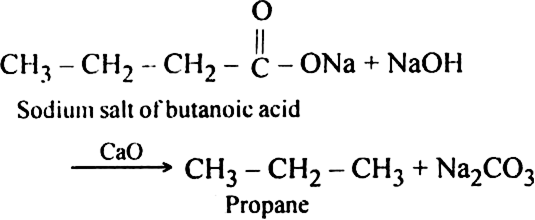
Sodium salt of which acid will be needed for the preparation of propane? Write chemical equation for the reaction.
What happens when:
(i) Propyne is heated with H2 in the presence of nickel at 473 K?
(ii) Water is dropped on aluminium carbide?
(iii) Sodium propionate is heated with sodalime?
(iv) Acetic acid is treated with hydroiodic acid in the presence of red phosphorus at 420 K?
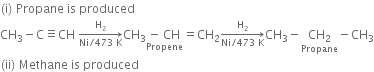
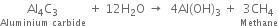
(iii) Ethane is produced
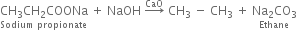
(iv) Ethane is produced

Assign reasons for the following:
(i) Boiling points of n-alkanes increase regularly with the increase in the number of carbon atoms.
(ii) Branched-chain alkanes have lesser boiling points than the straight chain alkanes.
(i) Alkanes are non-polar molecules and hence are held together by weak Vander Waal’s forces of attraction amongst their molecules. These forces act on the surface of molecules and their magnitude increases with the increase in surface area of the molecules. Thus, with the increase in the number of carbon atoms, the magnitude of Vander Waals forces increases and with that boiling points also increase.
(ii) This is because the branching of the chain makes the molecule more compact and bring the various atoms closer. As a result, the molecular size decreases. This decreases the surface area and hence the magnitude of Vander Waal’s forces (inter-particle forces) and lead to the decrease in boiling point. The boiling points of isomeric alkanes are given below: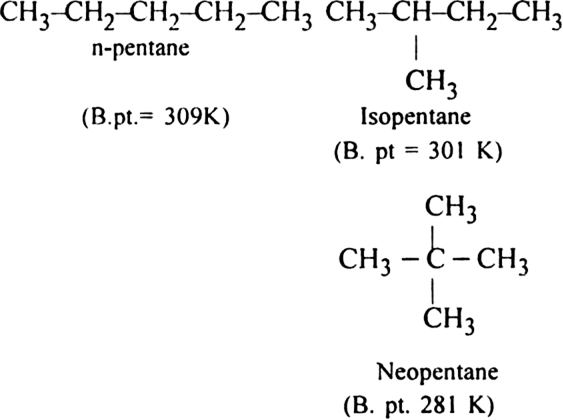
What effect does branching of an alkane chain has on its boiling point ?
Branching of an alkane chain makes the molecules more compact and brings various atoms closer. As a result, the molecular size decreases. This decreases the surface area and therefore the magnitude of Vander Waal's forces also decreases. Hence the boiling point of the alkane decreases with branching.
Assign reasons for the following:
(i) All C-H bonds in methane are equivalent.
(ii) Alkanes are called paraffins.
(i) This is because the carbon atom in methane is sp3 hybridised. Since all orbitals (four) in carbon have equivalent energies and shapes, therefore, they form all equivalent C-H bonds.
(ii) Alkanes are called paraffin because they are little reactive chemically due to the presence of strong and stable C-C bonds, therefore, alkanes take part in chemical reactions only under suitable conditions and are called paraffin.
Alkanes with even carbon atoms have higher melting points than alkanes with an odd number of carbon atoms.Explain.
This is because alkane chains with an even number of the carbon atoms (more symmetrical) pack more closely than those with an odd number of carbon atoms (less symmetrical) in the crystalline state. 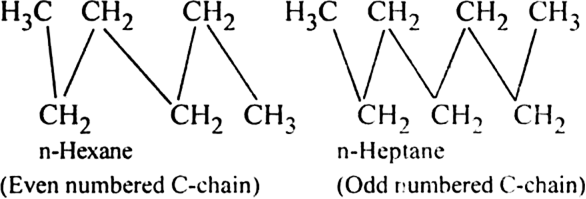
As a result, attractive forces between the individual chains of an alkane with an even number of carbon atoms are greater and thus the melting point is higher.
Describe substitution reaction with special reference to halogenation of alkane.
Substitution reaction: A substitution reaction is that which involves the direct replacement of hydrogen atoms of some other groups of a molecule by suitable atoms or groups without changing the structure of remaining part of the molecule. The product obtained is known as substitution product and new atom or group which enters the molecule is known as a substituent.
Halogenation: It involves the replacement of one or more hydrogen atom(s) of alkane by the corresponding number of a halogen atom(s).
(i) Chlorination: Chlorination of alkanes is carried out by treating alkane with chlorine in the presence of ultraviolet light or at 523-673 K temperature.
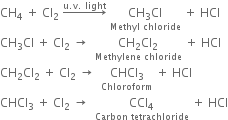
(ii) Bromination: Bromination takes place similarly but not so rapidly.
(iii) Iodination: Iodination is extremely slow and reversible due to the reducing nature of HI.
Thus, iodination is carried out in the presence of some oxidising agent like iodic acid (HIO3) of nitric acid (HNO3) which converts HI to iodine and pushes the reaction in the forward direction.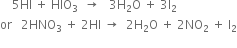
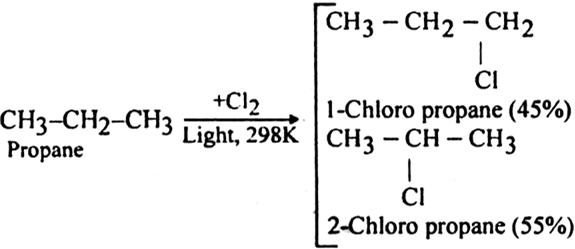
Halogenation of higher alkanes (ethane, propane etc), yields a mixture of all possible isomerism products. 
Why does the iodination of methane require an oxidising agent while so much reagent is needed in the chlorination and bromination of methane?
Iodine reacts with methane molecules reversibly. In fact, hydrogen iodide formed is a very strong reducing agent and it can convert iodomethane back to methane.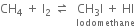
To overcome this difficulty, iodination is generally carried out in the presence of strong oxidising agents like iodic acid (HIO3) which oxides HI formed during the reaction to iodine. 
On the other hand, during the chlorination or bromination of methane, no such reducing agent is produced.
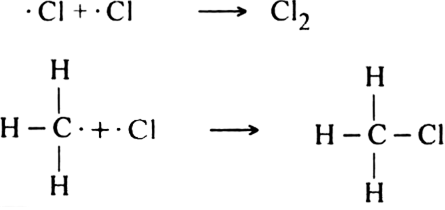
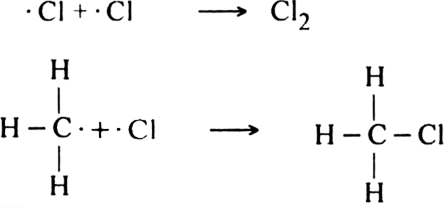
What are the products of chlorination of methane ? Describe the mechanism of the formation of each?
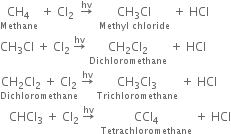
Mechanism: It involves free radical mechanism and consists of three steps:
(i) Chain initiating step: The chlorine molecule takes up energy either from ultraviolet light or from heat to form two chlorine free radicals by homolysis.

(ii) Chain propagating step: The free radical Cl collides with methane molecule to form methyl free radical.
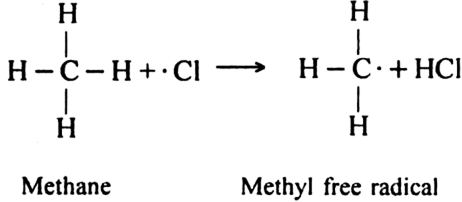
The above reaction is exothermic. The methyl free radical then attacks with chlorine molecule to form methyl chloride and chlorine free radicals.
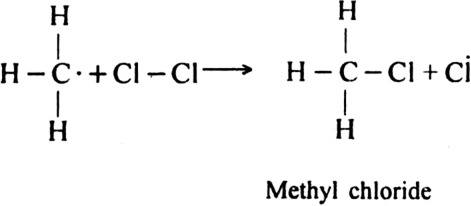
Formation of other chlorinated products:
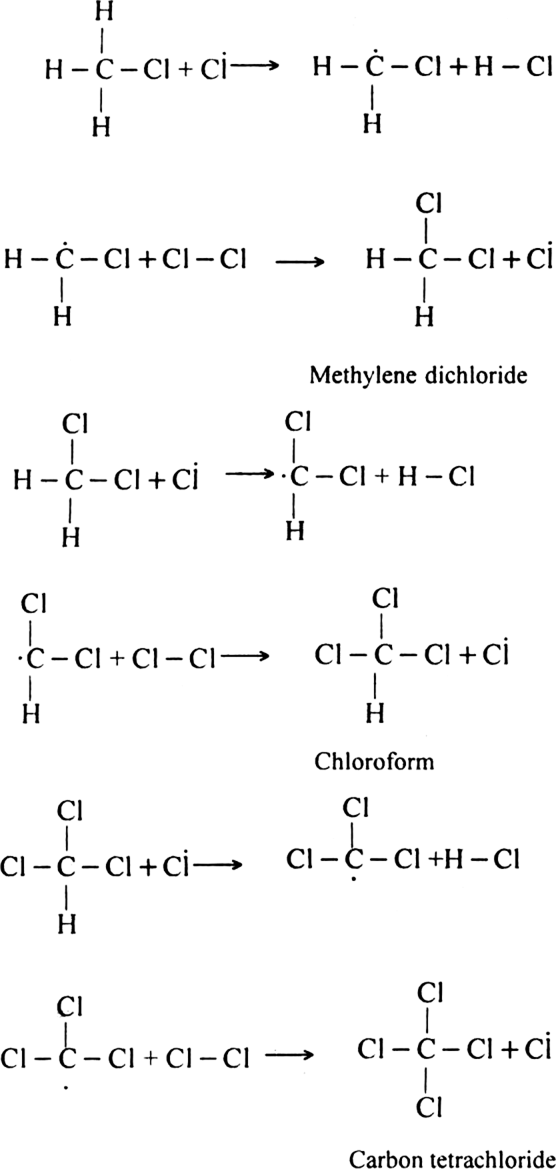
(iii) Chain terminating step: The reaction terminates when the free radical combines with each other.
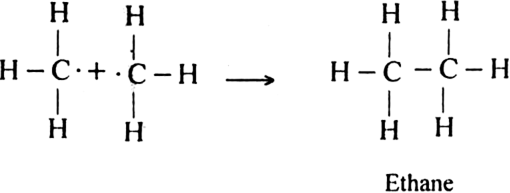
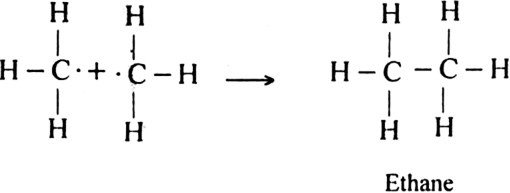
How do you account for the formation of ethane during chlorination of methane?
Chlorination of methane is a free radical reaction which occurs by the following mechanism:
3
(a) Chain initiating step:
(b) Chain propagating step.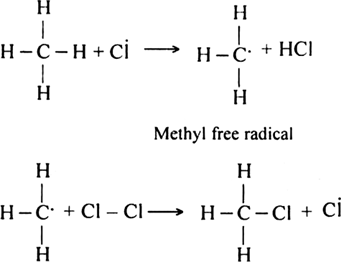
(c) Chain terminating step: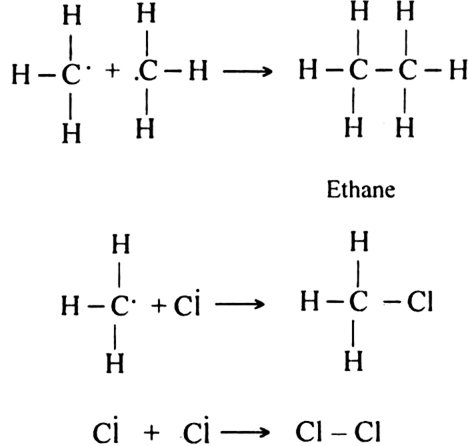
Since during the chain propagating step, CH3 free radicals are produced. This two methyl free radicals combine together in the chain-terminating step to form an ethane (CH3 - CH3) molecule.
Discuss:
(a) Nitration of an alkane.
(b) Sulphonation of alkane.
(a) Nitration of alkane: The process which involves the replacement of hydrogen atom of alkanes by nitro group (-NO2) is known as nitration of an alkane. It can be carried out in two ways:
(i) Liquid phase nitration; In this method higher alkane is heated with fuming HNO3 at 413 K under pressure.
(ii) Vapour phase nitration: Lower member of alkanes can be nitrated by vapour phase nitration i.e. by heating a gaseous mixture of hydrocarbon and nitric acid vapours at 673-773 K.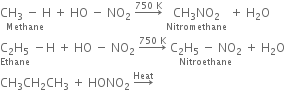
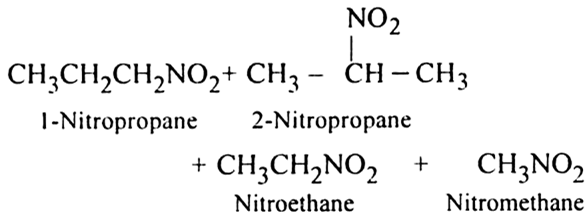
Mechanism of nitration:
The nitration of alkanes proceeds by the free radical mechanism.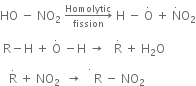
(b) Sulphonation of alkane: The process which involves the replacement of hydrogen atom of alkanes with a sulphonic acid group (-SO3H) is known as sulphonation of alkane.
It is carried out by heating higher alkanes (hexane or higher members) with fuming sulphuric acid.
Mechanism of sulphonation:
The sulphonation of alkanes proceeds by the free radical mechanism.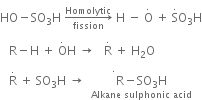
What do you mean by isomerization of alkanes?
Isomerisation of alkanes involves the conversion of straight-chain alkanes into isomeric branched chain alkanes.
For example, when butane is heated with aluminium chloride in the presence of dry HCl gas at 573K under about 35 atmospheres pressure, 2-methyl propane is produced.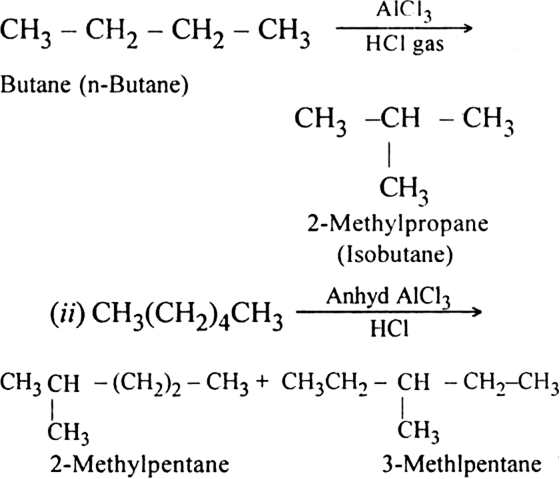
Explain briefly the process of cracking or pyrolysis.
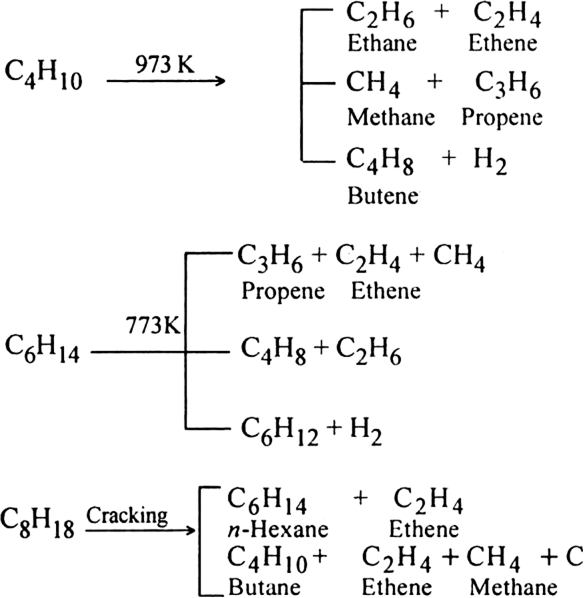
Pyrolysis of alkanes is supposed to occur by the free radical mechanism. Preparation of oil gas or petrol gas from kerosene oil or petrol involves the principle of pyrolysis. For example, heating to 973K in the presence of Pt, Pd or Ni gives a mixture of heptane and pentene.
(i) Thermal cracking: It may be carried out in the vapour phase or in the liquid phase. It is difficult to control and gives rise to complex product mixtures.
(ii) Catalytic cracking. It is carried out at a temperature of 670- 820 K using silica and alumina as a catalyst.
Catalytic cracking is useful as it gives gasoline having higher octane number.
The products formed during cracking depends upon:
(i) the structure of starting alkane
(ii) the pressure applied
(iii) use of a catalyst.
What are the products of chlorination of methane? Describe the mechanism of the formation of each?
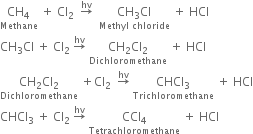
Mechanism: It involves free radical mechanism and consists of three types:
(i) Chain initiating step: The chlorine molecule takes up energy either from ultraviolet light or from heat to form two chlorine free radicals by homolysis.
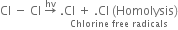
(ii) Chain propagating step: The free radical Cl collides with methane molecule to form methyl free radical.
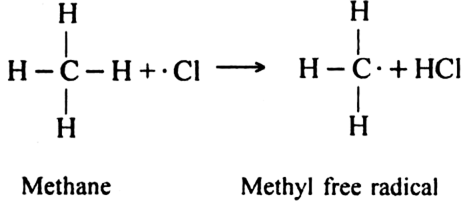
The above reaction is exothermic. The methyl free radical then attacks with chlorine molecule to form methyl chloride and chlorine free radical.
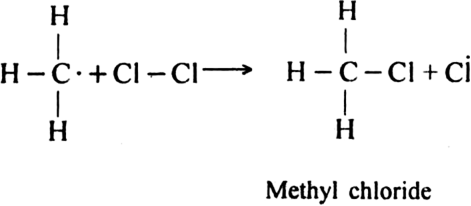
Formation of other chlorinated products:
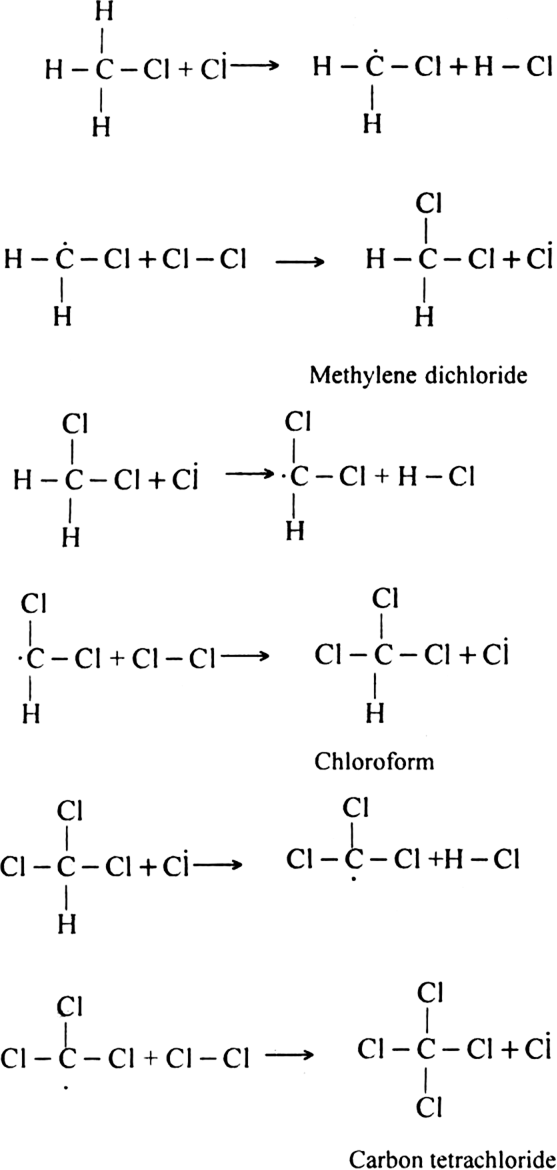
(iii) Chain terminating step: The reaction terminates when the free radical combines with each other.
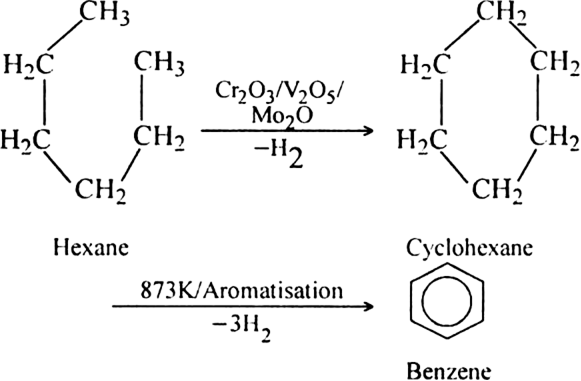
What is reforming (or aromatization)? Why is it done?
Reforming: It is a process of converting an alkane or cycloalkane with six or more carbon atoms into corresponding aromatic hydrocarbon under suitable conditions. The process which involves cyclisation and dehydrogenation is called aromatization. For example, aromatic hydrocarbons can be obtained:
(i) By catalytic dehydrogenation of cyclohexane and related compounds.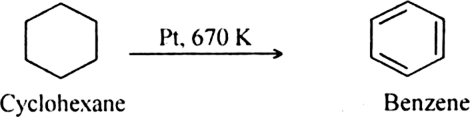
(ii) By cyclisation of alkanes (having six or more carbon atoms) to cycloalkane and then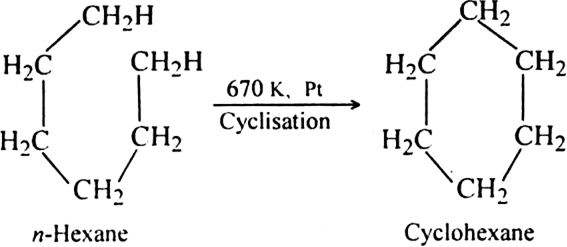
subsequent dehydrogenation at a higher temperature (≃ 670 K) in the presence of a catalyst such as platinum, palladium or nickel. For example,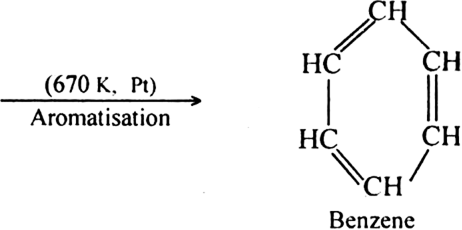
(ii) Using n-Heptane: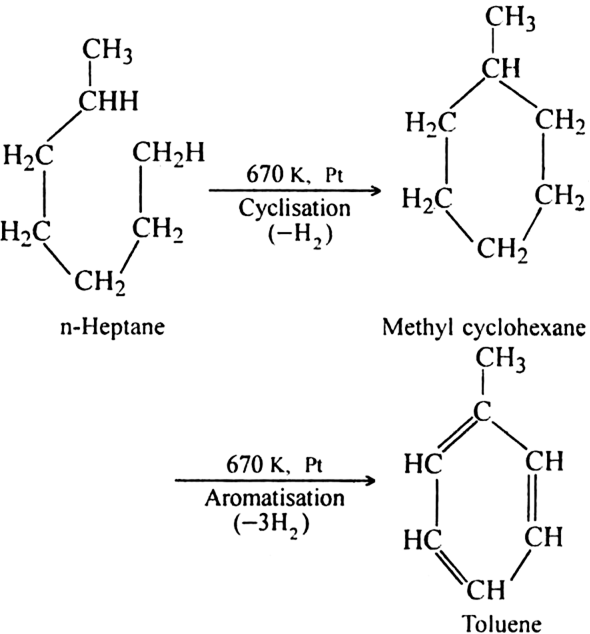
Platinum is the most suitable catalyst, therefore reforming is also called platforming.
Purpose.
(i) It is done to increase the efficiency of gasoline which acts as a fuel as aromatic hydrocarbons have been found to behave as better fuel than the corresponding aliphatic or cyclic hydrocarbons.
(ii). It is also used to prepare aromatic hydrocarbons such as benzene, toluene, o-xylene etc. from alkanes and cycloalkanes.
Define the terms conformation and conformational isomerism.
The conformation of a molecule refers to different three-dimensional positions of atoms or groups relative to each other, arising from rotation about single (σ) bonds. The energy required for rotation about C-C bond or a bond is very low and such rotations usually occur readily. So, the various conformations of a molecule are freely interconvertible. This phenomenon of getting different conformations arising from rotation about a single or sigma bond is called conformational isomerism.
Define the terms conformation and conformational isomerism.
The conformation of a molecule refers to different three-dimensional positions of atoms or groups relative to each other, arising from rotation about single (σ) bonds. The energy required for rotation about C-C bond or a bond is very low and such rotations usually occur readily. So, the various conformations of a molecule are freely interconvertible. This phenomenon of getting different conformations arising from rotation about a single or sigma bond is called conformational isomerism.
Discuss the conformation in the molecule of ethane.
Let us fix one carbon atom and allow the other carbon atom to rotate. By doing so, we get an infinite number of arrangements (conformations) which differ in the spatial arrangements of hydrogen atoms bonded to each carbon atom. Out of these infinite number of conformations, the two are most important:
1. Eclipsed conformation (cis): In this conformation, the hydrogen atoms attached to one carbon atom completely cover or eclipse the hydrogen atoms attached to the other carbon atom in space. Consequently, the repulsion in these atoms is maximum and the conformation has maximum energy and less stable.
2. Staggered conformation (trans, anti-form): In this conformation, the hydrogen atoms attached to the carbon atoms are maximum apart in space and the repulsion in them is minimum. Thus, the staggered conformation has the least energy and more stable.
The eclipsed and staggered conformation of ethane are represented with the help of space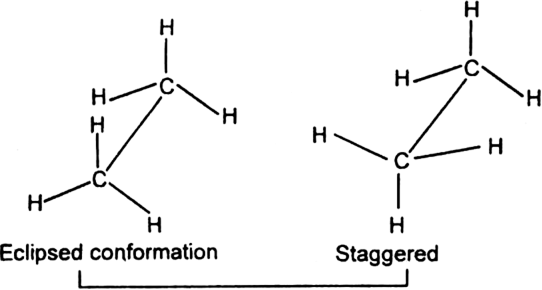
models (3D or three-dimensional separation) called saw horse models.
Newman has represented the eclipsed and staggered conformation of ethane with the help of Newman projections.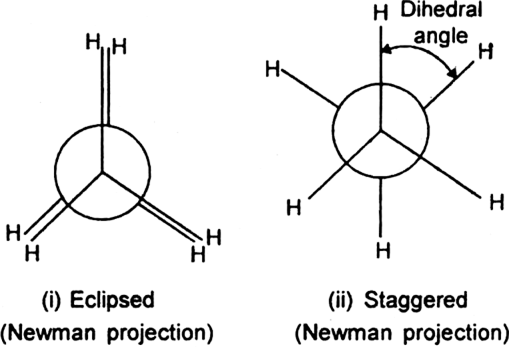
Out of staggered and eclipsed conformations of ethane, which is more stable and why ?
Staggered conformation of ethane is more stable than the eclipsed conformation.
Reason. When the staggered conformation is rotated through an angle of 60°, it changes to eclipsed conformation and the eclipsed conformation when further rotated through 60° gives back the staggered conformation.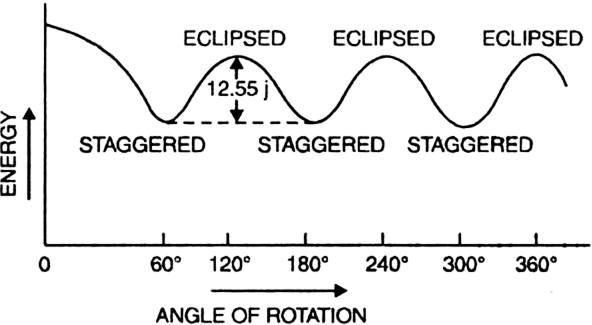
These two conformations differ in their energy contents and also in their relative stabilities. The staggered conformation with minimum repulsions between C-H bonding electrons and hydrogens of one methyl group and those of other has lesser energy than the eclipsed conformation where the force of repulsion between C-H bonding electrons and hydrogen of one methyl group and those of other is maximum.
The energy difference between staggered and eclipsed conformation of ethane is 12-55 kJ/mole. Hence staggered conformation is more stable than the eclipsed conformation.
Sponsor Area
Can eclipsed and staggered conformations of ethane be isolated? Give reasons.
No, because the difference of energy between these two conformations is very small, so even at room temperature these two interconvert rapidly and hence cannot be isolated.
What are alkenes ?
 in their molecules. The alkenes are also called olefins because the lower gaseous members from oily products with chlorine or bromine. The double bond in an alkene is also known as an Ethylenic double bond or Olefinic bond. The general formula of alkene is CnH2n where n = 2,3, 4...... etc. Since there can be no alkene having one carbon (due to the absence of >C = C< double bond), the first member of the series in bond), the first member of the series is C2H4. It is commonly known as ethylene.
in their molecules. The alkenes are also called olefins because the lower gaseous members from oily products with chlorine or bromine. The double bond in an alkene is also known as an Ethylenic double bond or Olefinic bond. The general formula of alkene is CnH2n where n = 2,3, 4...... etc. Since there can be no alkene having one carbon (due to the absence of >C = C< double bond), the first member of the series in bond), the first member of the series is C2H4. It is commonly known as ethylene.When n = 2 C2H4 Ethene (Ethylene)
n = 3 C3H6 Propene (Propylene)
What kind of structural isomerism is shown by alkenes ?
Alkenes show two types of structural isomerism:
(i) Chain isomerism: It is caused by different arrangements of carbons in the chain. For example.
(ii) Position isomerism: It is caused due to the different positions of a double bond in the alkene. For example 1-butene and 2-butene.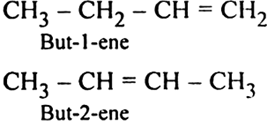
Calculate the number of sigma (σ) and pi  bonds in the following structures:
bonds in the following structures: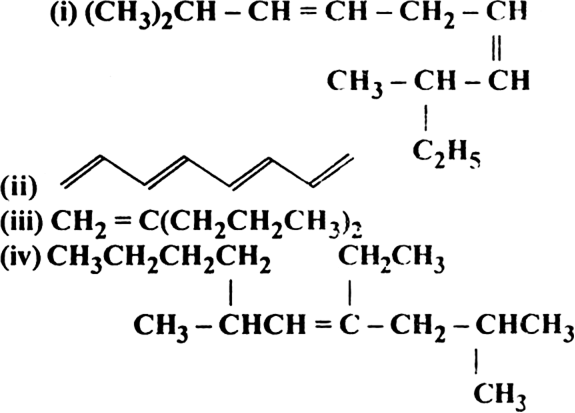
 bonds 2
bonds 2(ii) σ bonds 7,
 bonds 4
bonds 4(iii) σ bonds 23,
 bonds 1
bonds 1(iv) σ bonds 41,
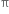 bonds 1
bonds 1
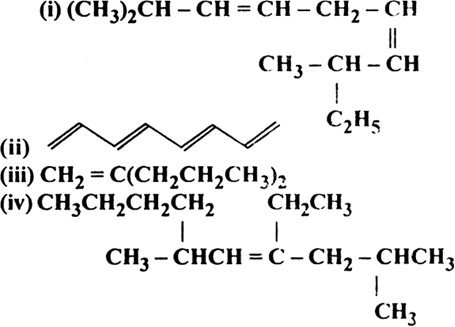
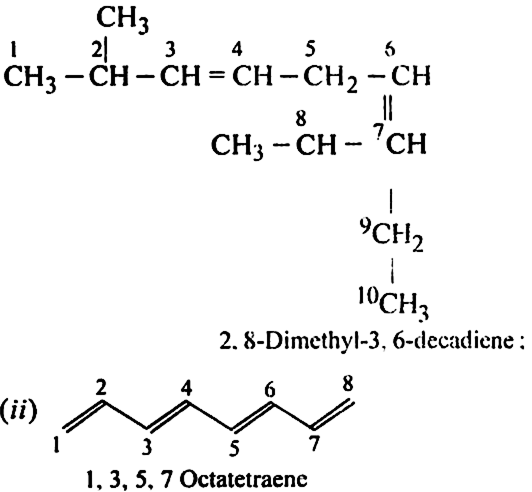
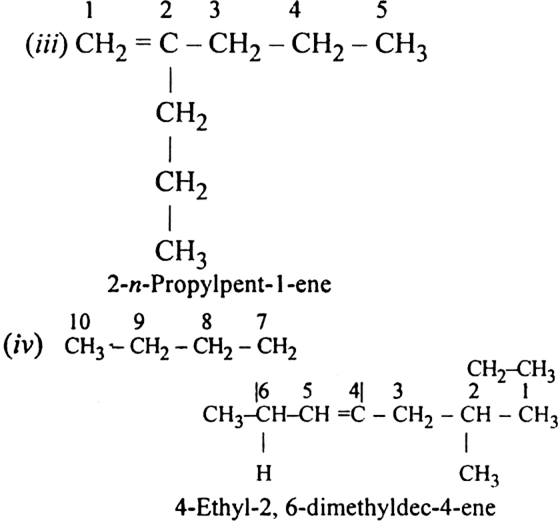
Write structure and IUPAC names of different structural isomers of alkenes corresponding to C5H10.
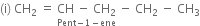

What is meant by hindered (or restricted) rotation around carbon-carbon double bond? What type of isomerism does it lead to?
Hindered rotation about double bond: The carbon-carbon double bond in ethylene and other compounds consists of a σ bond and a  bond. The
bond. The 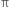 bond involves the overlapping of unhybridized p-orbitals of two carbon atoms above and below the plane of the atoms. Because of this type of overlapping, rotation around carbon-carbon double bond is strongly hindered and can do so only if the
bond involves the overlapping of unhybridized p-orbitals of two carbon atoms above and below the plane of the atoms. Because of this type of overlapping, rotation around carbon-carbon double bond is strongly hindered and can do so only if the 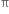 bond breaks. The breaking of the
bond breaks. The breaking of the 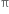 bond requires about 284 kJ of energy per mole which is not available to the molecule at room temperature. Hence, the atoms or groups attached to each carbon atom can not rotate about it. The rotation about the double bond is, therefore, restricted or hindered.
bond requires about 284 kJ of energy per mole which is not available to the molecule at room temperature. Hence, the atoms or groups attached to each carbon atom can not rotate about it. The rotation about the double bond is, therefore, restricted or hindered.
Isomerism due to hindered rotation: Due to hindered rotation around carbon-carbon double bond, the relative positions of groups attached to the double bonded carbon atoms get fixed. As a result, several alkenes can exhibit geometrical isomerism.
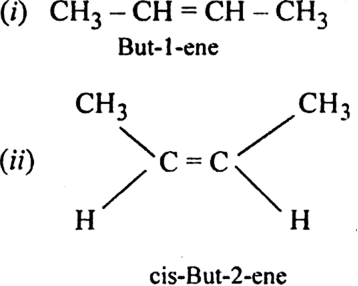
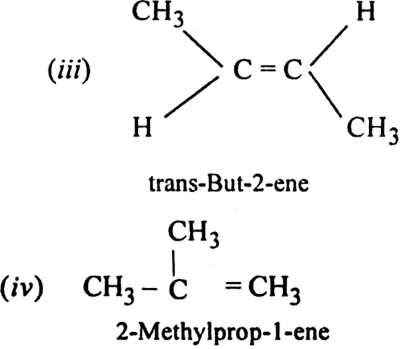
What is geometrical isomerism? What is its cause? Give examples.
Cause of geometrical isomerism: Geometrical isomerism is due to the restricted or hindered rotation around the carbon-carbon double bond. Due to the hindered rotation around carbon-carbon double bond, the relative positions of atoms or group attached to the doubly bonded carbon atoms get fixed. For example, the molecular formula C2A2B2with the structural formula BAC=CAB represents two geometrical isomers.
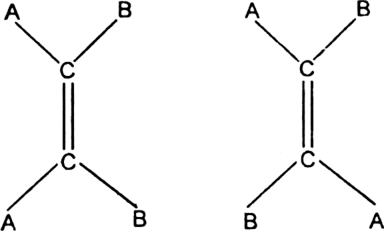
Isomer in which similar atoms or groups lie on the same side of the double bond is called cis-isomer. The geometrical isomer I represent a cis-isomer. Isomer in which the similar atoms or groups lie on the opposite sides of the double bond is called trans-isomer. The geometrical isomer (ii) represents a trans-isomer. Hence, geometrical isomerism is known as cis-trans isomerism e.g.
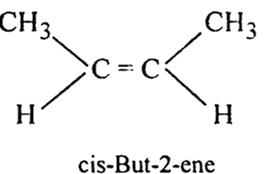
(i) But-2-ene exhibits geometrical isomerism.
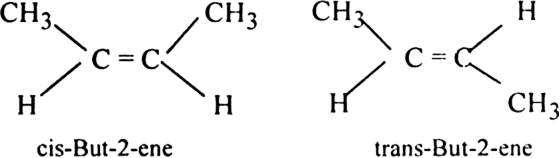
(ii) Hex-3-ene shows geometrical isomerism.
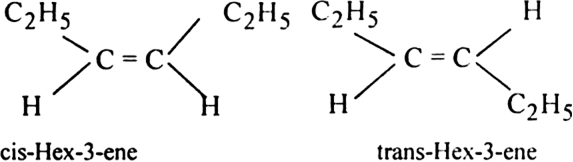
(iii) 1, 2-dichloroethene exhibits geometrical isomerism.
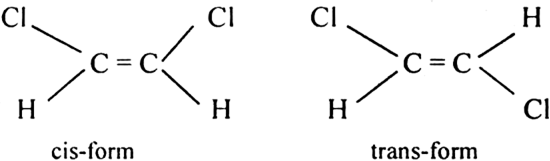
What are the necessary and sufficient conditions for a compound to exhibit geometrical isomerism ?
Conditions for geometrical isomerism: There are two necessary conditions for a compound to possess geometrical isomerism:
(i) It must contain a carbon-carbon double bond in the molecule.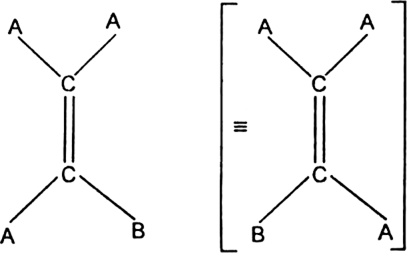
(ii) Two unlike atoms or groups must be linked to each doubly bonded carbon atoms.
Geometrical isomerism among alkenes does not occur when the doubly bonded carbon carry identical atoms or groups. For example, AAC = CAB does not exhibit geometrical isomerism.
Name the various structural isomers possible in C4H8. Which one will exhibit geometrical isomerism?
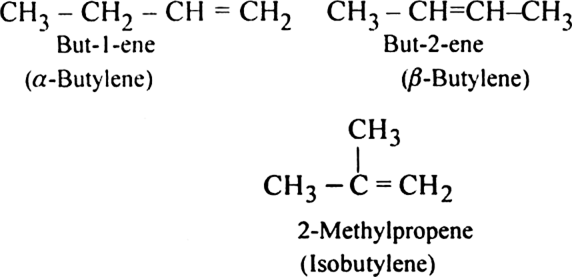
Only But-2-ene exhibits geometrical isomerism. It's two isomers are:
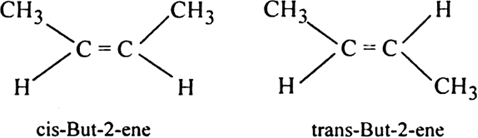
Alkanes and alkynes do not exhibit geometrical isomerism. Explain.
Alkanes contain carbon-carbon single bond and there is free rotation around single bond or sigma bond. Alkynes contain triple bond around which the rotation is hindered but the molecule is linear. Therefore, the question of fixed arrangement does not arise. Hence alkanes and alkynes do not show geometrical isomerism.
How will you explain that there exists two varieties of 1, 2,-dichloroethene while there is only one type of 1, 2-dichloroethane?
The structure of 1, 2-dichloroethene is![]()
Since in the above structure there is C = C bond and two chlorine atoms are present at two different carbon atoms, therefore, it is capable of exhibiting geometrical isomerism. The two geometrical isomers (i.e. two varieties) of the above compound can be represented as: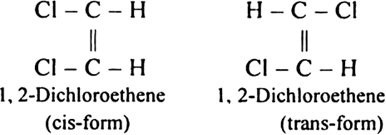
The structure of 1, 2-dichloroethane is![]()
The molecule has C-C bond. As the rotation of the atoms or groups about single bond is quite free, the compound fails to show geometrical isomerism.
Which of the following compounds would show geometrical isomerism? Draw the isomeric structures to support your answer:
(i) But-2-ene
(ii) Pent-1-ene
(iii) 2-Methyl-But -2-ene
(iv) 3, 4-Dimethyl-hex-3-ene
The compounds (i) 2-Butene and (iv) 3, 4-Dimethyl-3-hexene would show geometrical isomerism. In both the cases, the two atoms or groups attached to the double bonded carbon atoms are different.
(i) But -2- ene (CH3 - CH = CH - CH3) exhibits geometrical isomerism.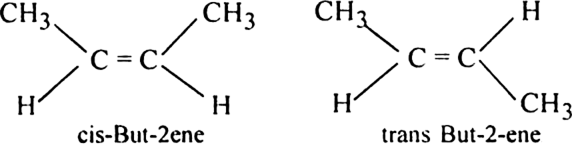
(iv) 3, 4-Dimethylhex-3-ene: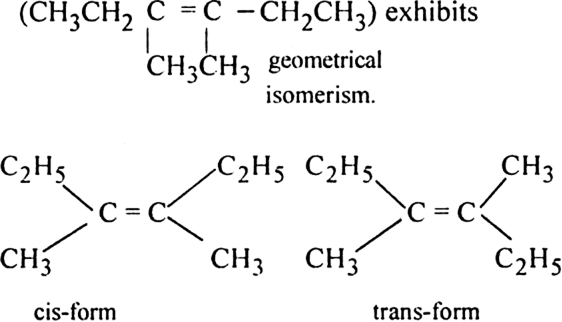
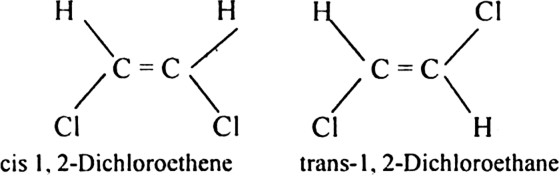
Draw cis and trans isomers of the following compounds. Also, write their IUPAC names:
(i) CHCl = CHCl
(ii) C2H5CCH3 = CCH3C2H5
(ii) cis and trans isomers of C2H5CCH3 = CCH3C2H5 are as follows:
Which of the following compounds will show cis-trans isomerism?
(i)(CH3)2C = CH-C2H5
(ii) CH2 = CBr2
(iii) C2H5CH = CH-CH2
(iv) CH3CH = CClCH3
Draw the cis and trans structures of hex-2-ene. Which isomer will have higher b.p. and why?
Hex-2-ene has the following cis and trans structures:
cis isomer possesses higher boiling point due to the greater magnitude of dipole-dipole interactions as compared to the trans isomer.
Out of the two - trans-but-2-ene and cis-but-1-ene, which is more stable and why
Trans-but-2-ene has less heat of hydrogenation than the cis-isomer, therefore it is more stable. 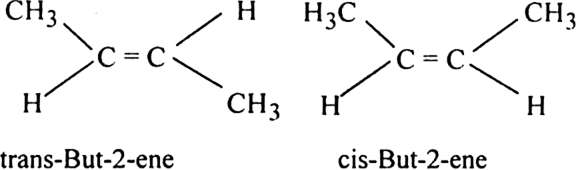
In the trans isomer, the two methyl groups are present on the opposite side as compared to cis-isomer. As a result, the Vander Waal's forces of attraction in trans isomer are less. This means that the trans isomer has less heat of hydrogenation and is more stable than the cis-isomer.
What happens when 2-Bromobutane is heated with alcoholic KOH. Account for the product formed.
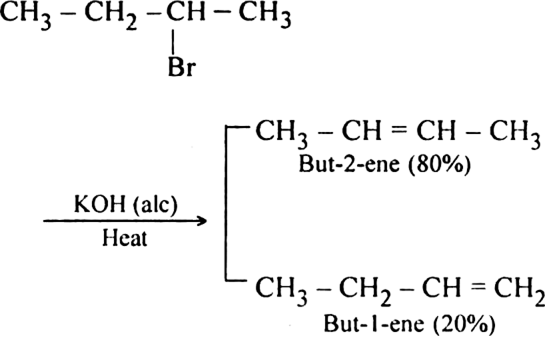
Formation of but-2-ene as the major product can be explained by Saytzeff's rule which states that if an alkyl halide undergoes elimination in two different ways, then the more highly substituted alkene i.e. having lesser number of hydrogen atoms on the doubly bonded carbon atoms, is the major product of dehydrohalogenation reaction.
What is Saytzeff’s rule? Explain it with an example.

What happens when butan-2-ol is heated with concentrated H2SO4. Account for the product formed.
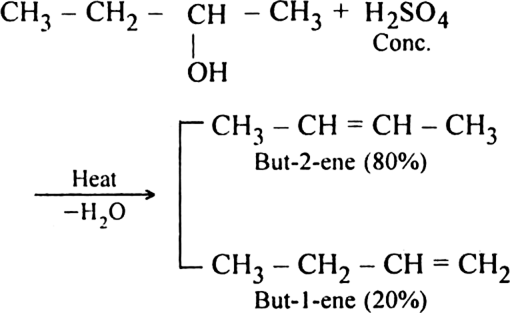
What happens when:
(i) Ethyl alcohol is heated in the presence of Al2O3 at 493 K?
(ii) Ethylene dibromide is heated with zinc dust?

(ii) Ethylene is formed by dehalogenation reaction.
Explain the mechanism of the following reaction: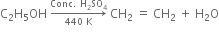
Mechanism of the above reaction involves three steps:
(i) Formation of protonated alcohol. H2SO4 ionises to give H+ ion which attacks the molecule of alcohol to form protonated alcohol.
(ii) Formation of the carbocation. Protonated alcohol eliminates a molecule of water to give a carbocation. 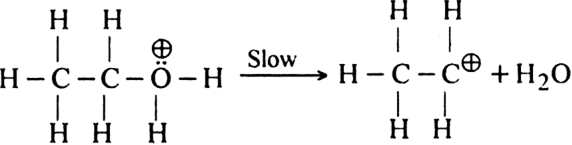
(iii) Formation of ethylene. Carbocation finally loses a proton to hydrogen sulphate ion to form ethylene. 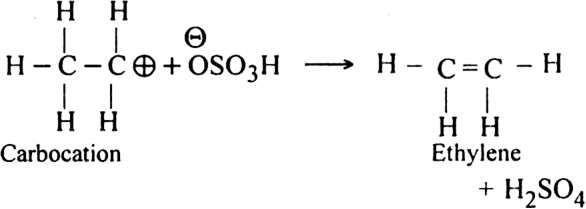
How are alkenes prepared from Alkynes ?
Alkenes are prepared by the partial reduction of alkynes.
(a) Using Lindlar’s catalyst. Catalytic reduction of alkynes in the presence of palladium catalyst deposited over BaSO4 or CaSO4 and partially poisoned by S or quinoline (Lindlar’s catalyst) predominantly gives us alkenes.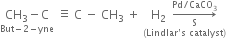
(b) Using Birch reduction. If alkynes are reduced by sodium and liquid ammonia (Birch reduction), trans-alkenes are major products. 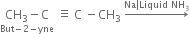
The 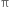 -bond is weaker than carbon-carbon σ bond. Explain.
-bond is weaker than carbon-carbon σ bond. Explain.
The >C = C< bond is made of σ bond and 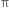 bond. The
bond. The 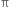 bond is weaker than σ bond because electron in the
bond is weaker than σ bond because electron in the  bond are more diffused in space i.e. orbitals participating in the
bond are more diffused in space i.e. orbitals participating in the 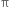 bond formation overlap sidewise only to small extent. On the other hand, orbitals participating in the σ bond formation overlap axially to a greater extent.
bond formation overlap sidewise only to small extent. On the other hand, orbitals participating in the σ bond formation overlap axially to a greater extent.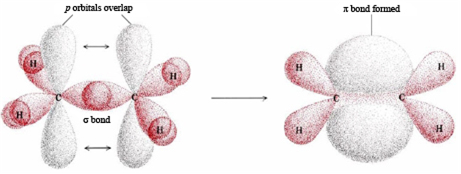
The greater the extent of overlapping, the higher the probability of finding the valence electrons in between the nuclei and hence the bond will be stronger and shorter. Hence overlap in 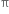 bond is less effective as compared to σ bond.
bond is less effective as compared to σ bond.
Why are alkenes more reactive than alkanes ?
Alkenes have a carbon-carbon double bond (>C = C<) in their molecules. The >C = C< bond is made up of one strong σ bond and one weak 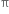 bond. The electrons of the
bond. The electrons of the 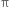 bond are more exposed which increase reactivity. In other words, the presence of
bond are more exposed which increase reactivity. In other words, the presence of 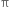 bond (formed by the sideways overlapping of atomic orbital) makes alkenes highly reactive chemically as compared to alkanes.
bond (formed by the sideways overlapping of atomic orbital) makes alkenes highly reactive chemically as compared to alkanes.
Why do alkenes undergo electrophilic addition reactions?
Alkenes undergo electrophilic addition reaction. The 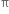 electrons constituting the
electrons constituting the 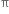 bond in the double bond are more mobile, easily detachable and more easily available for the chemical reaction. This means >C = C< can act as a source of electrons. There are certain reagents (electrophiles) which are capable of adding to alkene molecules forming an addition compound. Since such reactions are initiated by the electrophiles, hence are known as Electrophilic addition reactions.
bond in the double bond are more mobile, easily detachable and more easily available for the chemical reaction. This means >C = C< can act as a source of electrons. There are certain reagents (electrophiles) which are capable of adding to alkene molecules forming an addition compound. Since such reactions are initiated by the electrophiles, hence are known as Electrophilic addition reactions.
The mechanism proceeds in two steps:
(i) Electromeric effect and electrophilic attack.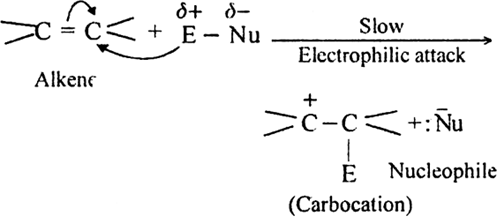
(ii) Attack of nucleophile. The nucleophile released in slow step combines with carbocation to give the addition product. 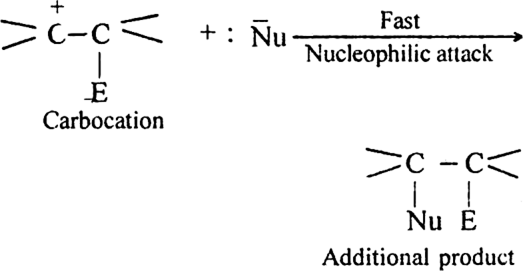
Discuss the mechanism of addition of bromine to ethylene.
Bromine adds to ethylene at ordinary temperature to form ethylene dibromide. 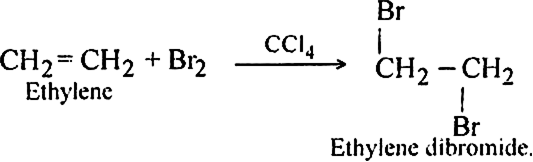
Mechanism: The mechanism of addition of bromine to ethylene is electrophilic in nature and consists of the following steps:
(i) Electromeric effect and electrophilic attack. 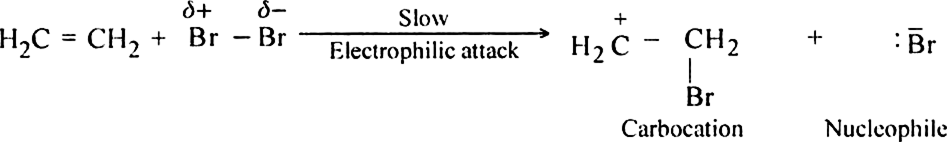
(ii) Attack of the nucleophile: The nucleophile released in the slow step combines with carbocation to give 1, 2-Dibromoethane. 
The above mechanism cannot explain the formation of the trans product. The formation of trans product can only be explained by cyclic halonium ion mechanism which consists of the following steps:
Step I. Formation of cyclic bromonium ion.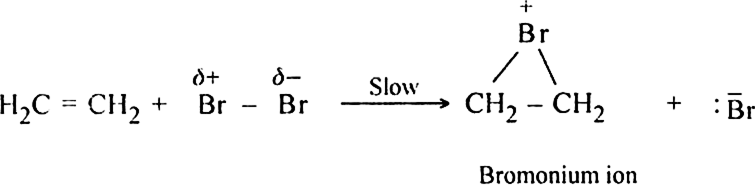
Step II. Formation of the trans product.
The Br- ion attacks one of the carbon atoms of bromonium ion (from the side opposite to that on which positively charged bromine is present) giving rise to the formation of trans addition product. 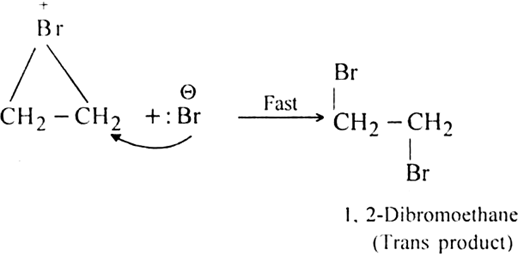
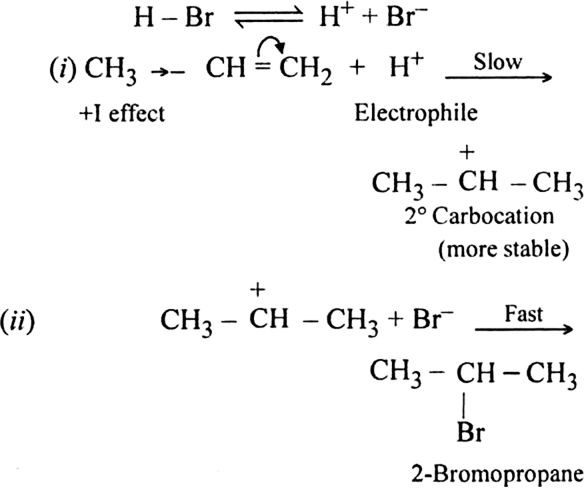
Discuss the mechanism of addition hydrogen acids to symmetrical alkenes. Justify the order of reactivity of halogen acids HI > HBr > HCl.
Alkenes react with halogen acids to form mono-haloalkanes called alkyl halides.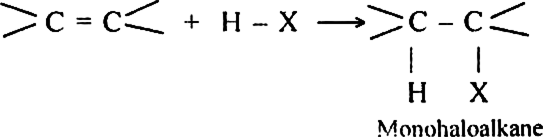
The order of reactivity of the halogen acids is HI > HBr > HCl.
The above order is justified on the basis of bond dissociation energies of the halogen acids.
HI (300 kJ mo-1) < HBr (365 kJ mol-1) < HCl (430 kJ mor-1) For example,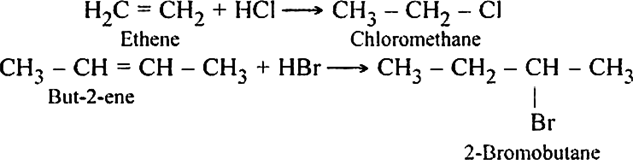
Mechanism: It is electrophilic addition reaction and consists of the following steps:
(i) Electromeric effect and electrophilic attack:
(ii) Attack of nucleophile: The nucleophile released in the slow step combines with carbocation to give chloroethane. 
Define Markownikoff's rule giving an example.
This rule states: In a normal addition, the negative part of the addendum (molecule to be added) adding to the double bond of an unsymmetrical alkene goes to that carbon atom which has lesser number of hydrogen atoms. Examples are: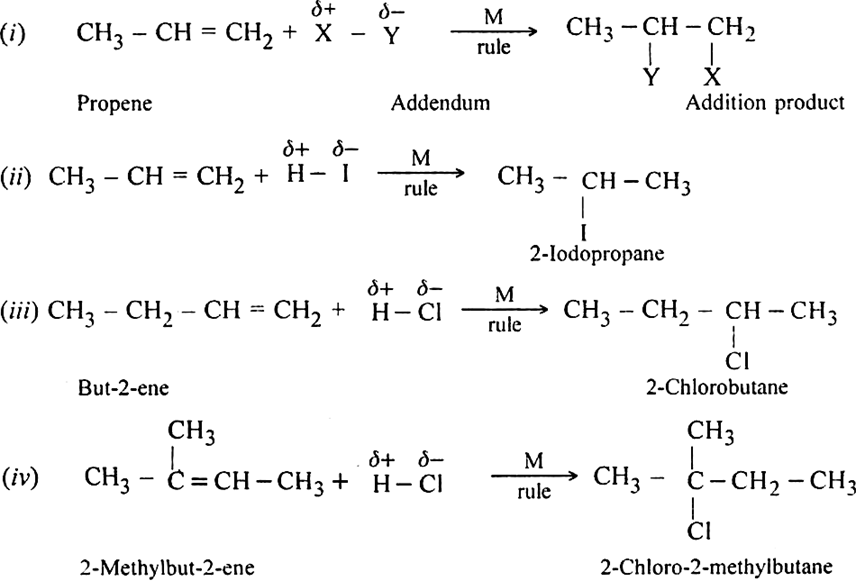
Give the mechanism of Markownikoffs rule as applied to unsaturated hydrocarbons.
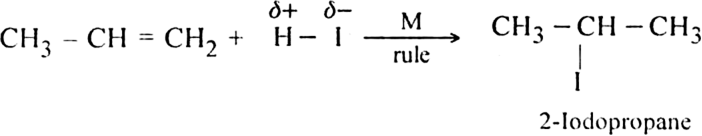
Mechanism: The reaction involves electrophilic addition. It is initiated by the attack of an electrophile (H+) on propene molecule. Two types of intermediate carbocations are formed.
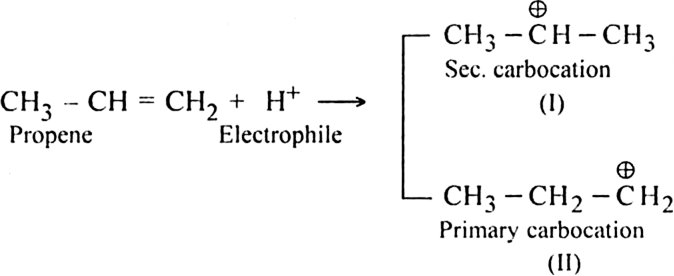
The carbocation which is mainly formed depends on upon its relative stability. Since secondary carbocation (I) is more stable than the primary carbocation (II), therefore, it would be formed faster and than in preference to carbocation (II).
The carbocation (I) then takes up the iodide ion
 of the HI to form isopropyl iodide as the main product.
of the HI to form isopropyl iodide as the main product. 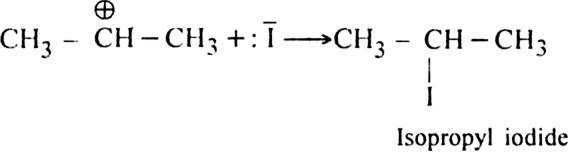
On the basis of above explanation. Markownikoffs rule may also be stated as The electrophilic addition to an unsymmetrical alkene always occurs through the formation of a more stable carbocation intermediate.
Discuss the free radical mechanism of addition of HBr to propene.
Or
Give the mechanism of addition of HBr to propylene in the presence of peroxide.
Propylene reacts with HBr in the presence of peroxide to form 1-bromo propane.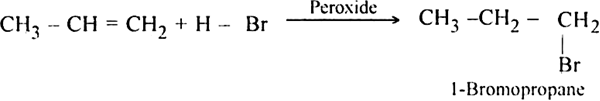
Mechanism: It proceeds with free radical mechanism and consists of the following steps:
Step I. Chain initiating step: Organic peroxide undergoes homolysis to from free radicals.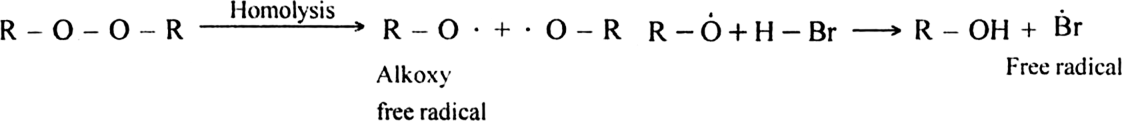
Step II. Chain propagating step: The bromine radical then adds to propene molecule in such a way that the more stable free radical is produced. 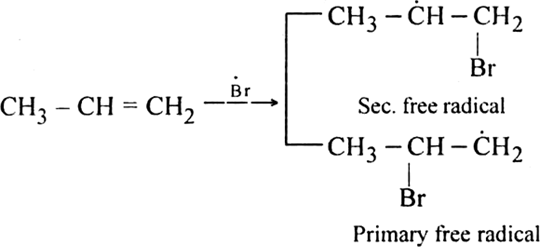
As the secondary free radical is more stable, it will be preferably formed. The secondary free radical then reacts with HBr to form 1-bromo-propane and bromine radical.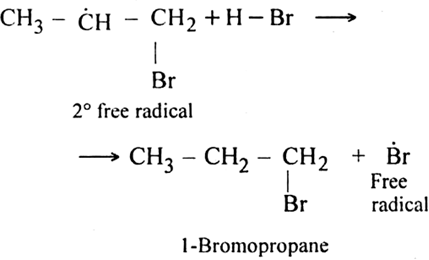
This bromine radical continues the chain by repeating the chain propagating step.
Step III. Chain terminating step: At the end, the reaction stops by coupling the radicals.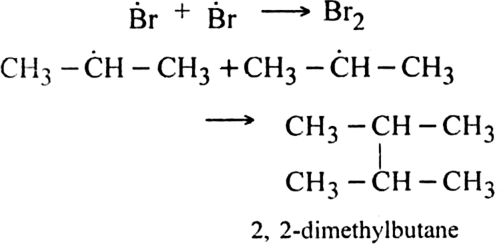
Write IUPAC names of the products obtained by addition reactions of HBr to hex-1-ene:
(i) in the absence of peroxide and
(ii) in the presence of peroxide.
(i) In the absence of peroxide. Hexene reacts with HBr in the absence of organic peroxide to form 2-Bromohexane. Addition takes place according to Markownikoffs rule.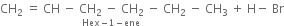
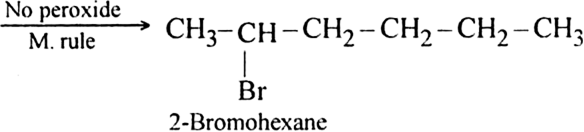
(ii) In the presence of peroxide. Hex-1-ene reacts with HBr in the presence of peroxide to form 1-bromohexane.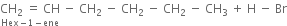
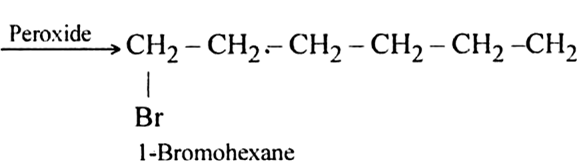
The addition of HBr to propene yields 2-broniopropane, while in the presence of benzoyl peroxide, the same reaction yields 1-bromopropane. Explain and give mechanism.
Formation of 1-Bromopropane:
In the presence of benzoyl peroxide, the addition of HBr to propene involves free radical mechanism in which Br-free radical is obtained by the action of benzoyl peroxide on HBr.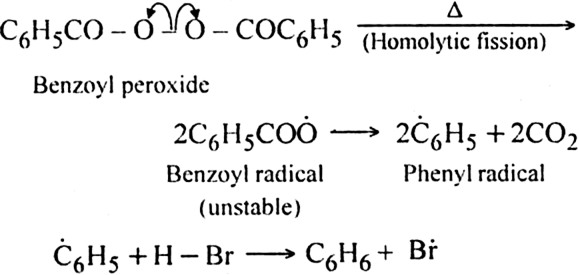
(i) Br radical adds to propene to form the more stable 2° free radical.
(ii) Free radical thus obtained rapidly abstracts a hydrogen atom from HBr to form 1-bromopropane.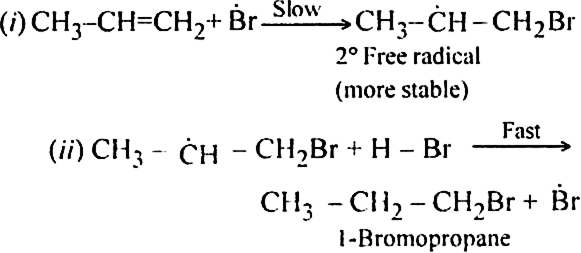
Why peroxide effect is shown only by HBr and not by HCl or HI?
Or
Explain why Kharasch effect is shown by HBr only and not by HCl or HI.
The mechanism of addition of HBr to a unsymmetrical alkene (say propene) in the presence of peroxide is free radical i.e. "H-Br undergoes homolysis to form free radical.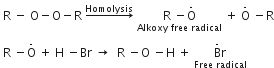
HCl is a very stable acid H-Cl bond (430 kJ moH) is stronger than H-Br bond (378 kJ mol-1) and is not broken symmetrically by the free radicals generated by peroxide. Hence the free radical addition of HCl to alkenes is not possible.
In the case of HI, the H - I bond (297 kJ mo-1) is weaker than H-Br bond and undergoes homolysis readily to form iodine free radical. But iodine free radicals have greater tendency to combine amongst themselves to form iodine molecules rather than add to the ethylenic bond.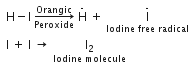
Hence HI does not respond to the peroxide effect.
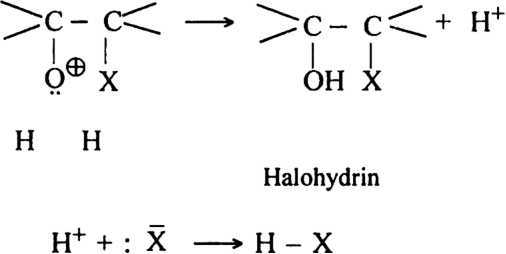
Discuss the mechanism of formation of halohydrin.
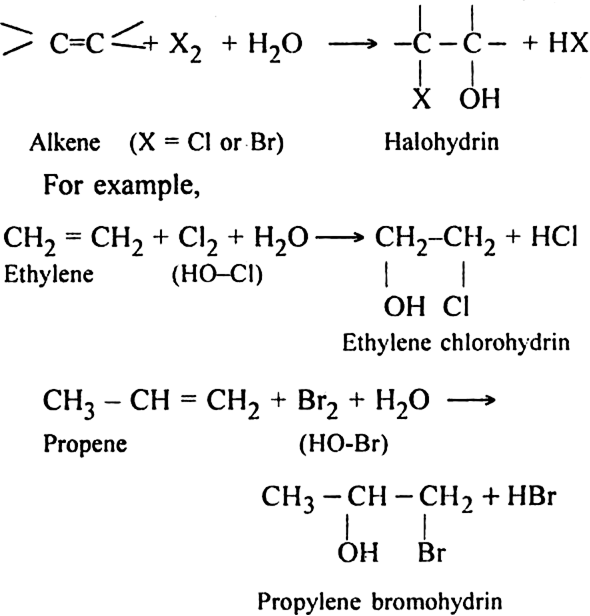
Mechanism. It is an electrophilic addition reaction involving the following steps:
Step I. Formation of carbocation: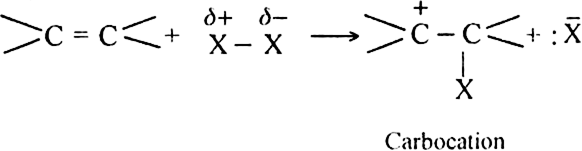
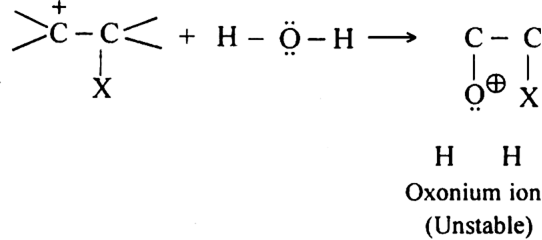
Step III. Loss of proton:
How do alkenes react with sulphuric acid? Discuss its mechanism.
Alkenes react with the sulphuric acid in cold to form alkyl hydrogen sulphates. The addition in case of unsymmetrical alkenes proceeds according to Markownikov's rule. 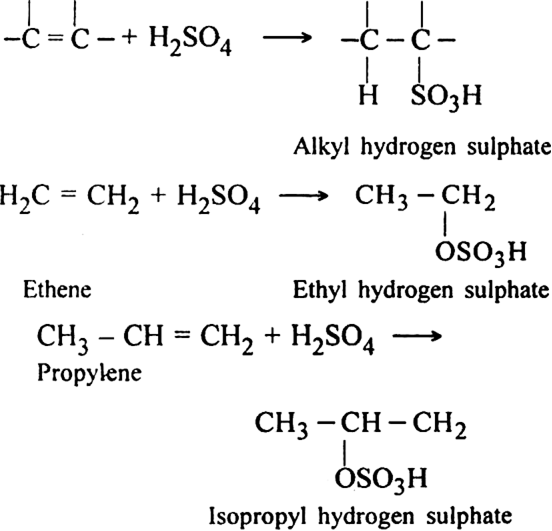
Mechanism: It involves electrophilic addition reaction and consists of the following two steps:
(i) Electromeric effect and electrophilic attack:![]()
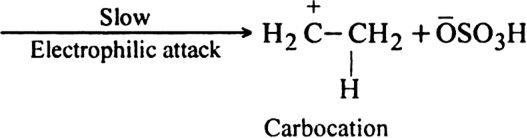
(ii) Attack of nucleophile: The nucleophile released in the slow step combines with carbocation to give ethyl hydrogen sulphate.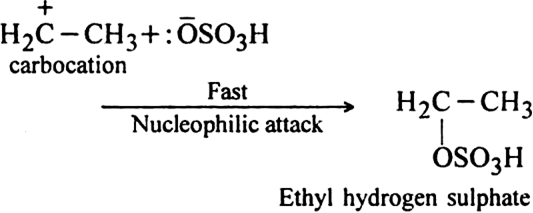
Write a short note on hydroboration oxidation.
Diborane adds to alkenes to give trialkyl borane which on oxidation gives alcohol.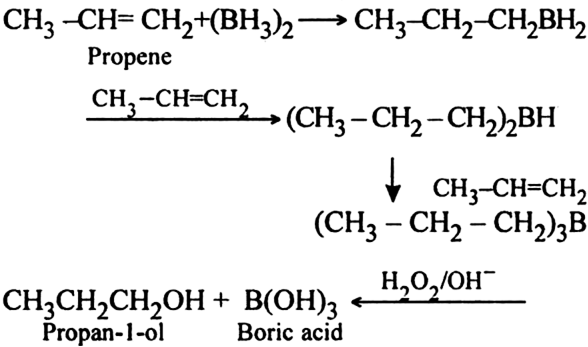
What is ozonolysis? What is its significance?
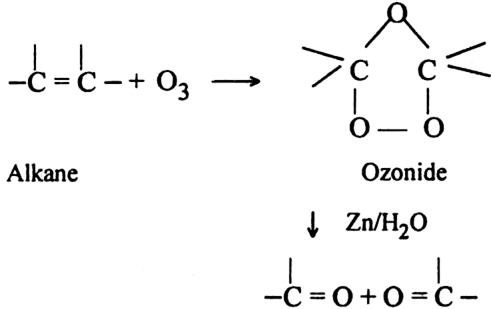
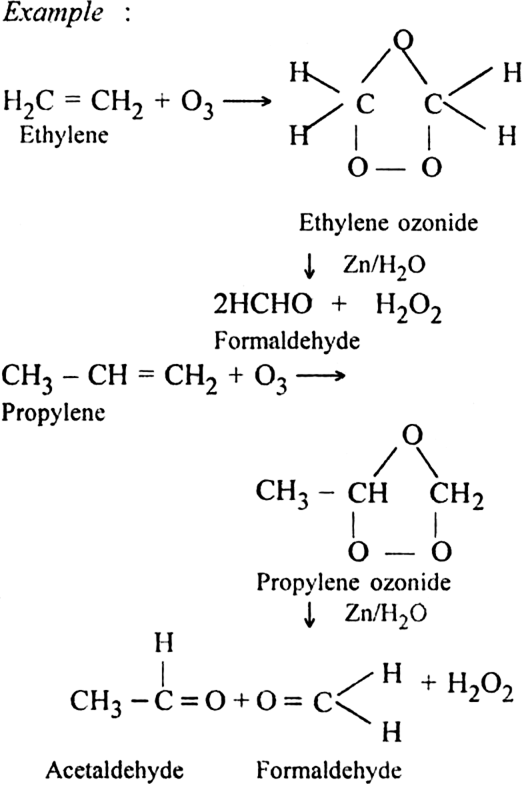
Significance: Ozonolysis is quite useful to locate the position of the double bond in an unknown alkene. This is done as shown below:
(i) Write the structures of the products (aldehydes or ketones or both) with carbonyl groups facing each other.
(ii) Remove oxygen atoms from carbonyl carbon and connect them by a double bond. For example, suppose the products of ozonolysis are ethanal and propanone.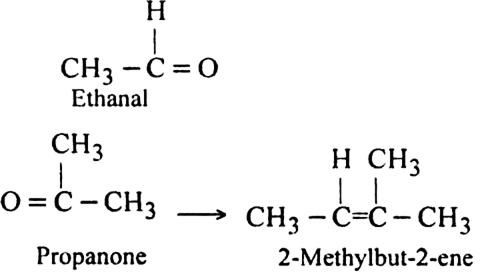
An alkene upon ozonolysis gives two isomeric compounds with molecular formula C3H6O. Write the structure of the compounds and also of the alkene.

An alkene 'A' on ozonolysis gives a mixture of ethanal and pentan-3-one. Write structure and IUPAC name of 'A'.
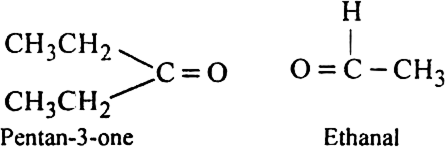
(ii) Remove the oxygen atoms and join the two ends by a double bond. Thus the structure of the alkene A is
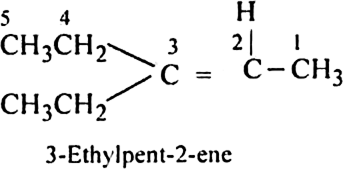
The above product we get as,
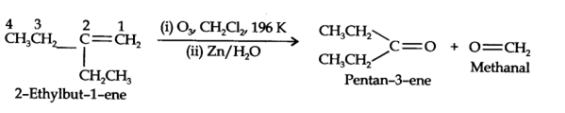
An alkene ‘A’ contains three C-C eight C-H bonds, one C - C  bond. ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 amu. Write the IUPAC name of ‘A’.
bond. ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 amu. Write the IUPAC name of ‘A’.
(i) An aldehyde having molar mass 44 amu is ethanal (CH3CHO).
(ii) Write two moles of ethanal side by side with their oxygen atoms facing towards each other.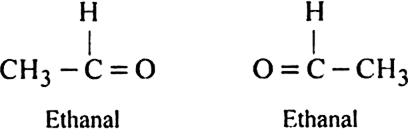
(iii) Remove the oxygen atoms and join the two ends by a double bond. 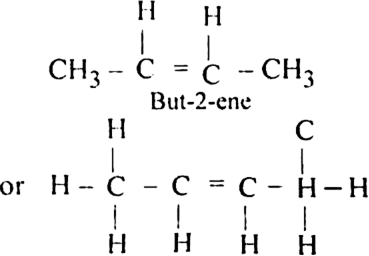
But-2-ene has three C-C, eight C-H and 
Propanal and pentan-3-one are the ozonolysis products of an alkene? What is the structural formula of the alkene?

(ii) Remove the oxygen atoms and join the two ends by a double bond. Thus the structure of the alkene is
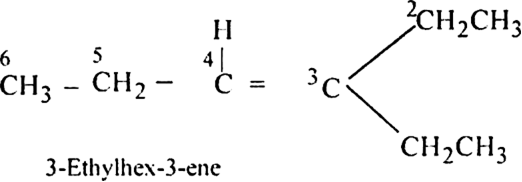
An alkene with molecular formula C7H14 gives propanone and butanal on ozonolysis. Write the structural formula.
 on ozonolysis gives propanone and butanal on ozonolysis. Join the product and removing the oxygen we get the parent alkene.
on ozonolysis gives propanone and butanal on ozonolysis. Join the product and removing the oxygen we get the parent alkene.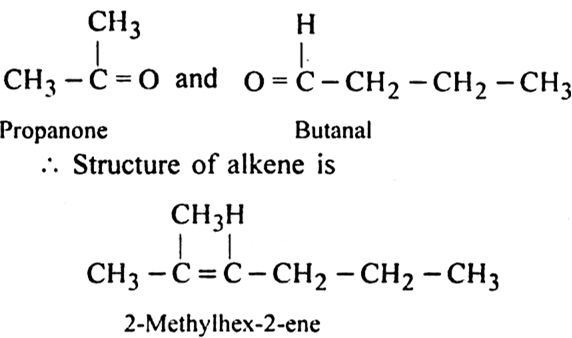
How does propene react with:
(i) Hydrogen
(ii) Bromine
(iii) Water in the presence of mineral acid
(iv) Chlorine water (or hypochlorus acid)?
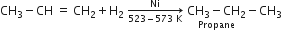
(ii) Action with bromine: Propene reacts with bromine (in CCl4) to form 1, 2-Dibromopropane.
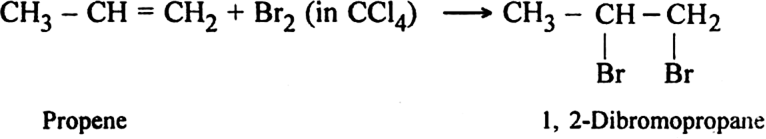
(iii) Action with water: Propane reacts with water in the presence of mineral acid to form propan-2-ol.
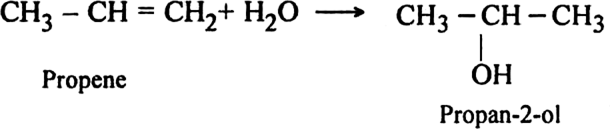
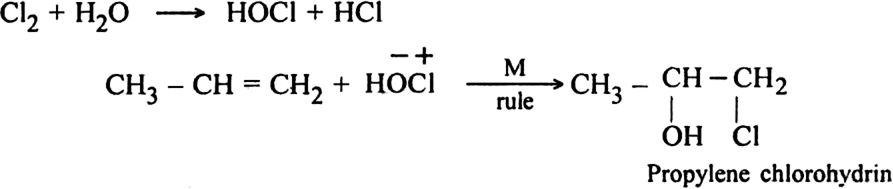
(iv) Action with chlorine water: Propene reacts with chlorine in the presence of water to form propylene chlorohydrin.
Discuss in brief the oxidation reaction of alkenes.
Different products are formed depending upon the nature of the oxidising agent.
(i) Using alkaline potassium permanganate solution (Baeyer’s reagent): Alkenes react with a cold dilute alkaline solution of KMnO4 to produce diols.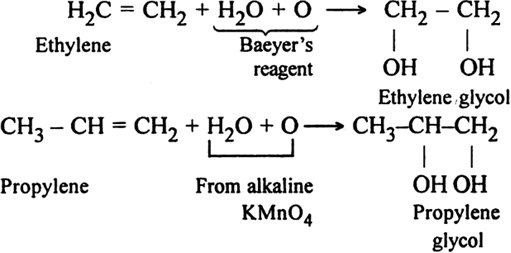
The pink colour of KMnO4 gets discharged and this reaction is known as Bayer's test for the detection of unsaturation in a compound.
(ii) Using hot KMnO4: Different products are formed depending upon the structure of alkene. In such reactions, the cleavage of C = C bond occurs.
In terminal alkenes: One of the product is always methanoic acid which is further oxidised to form CO2 and H2O.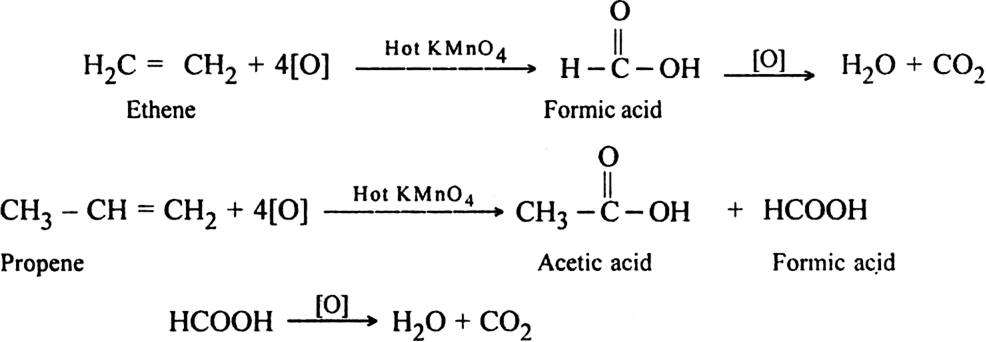
Note:= CH2 gets oxidised to CO2 and H2O.
In non-terminal alkenes. The products are carboxylic acids and/or ketones depending upon the nature of alkene.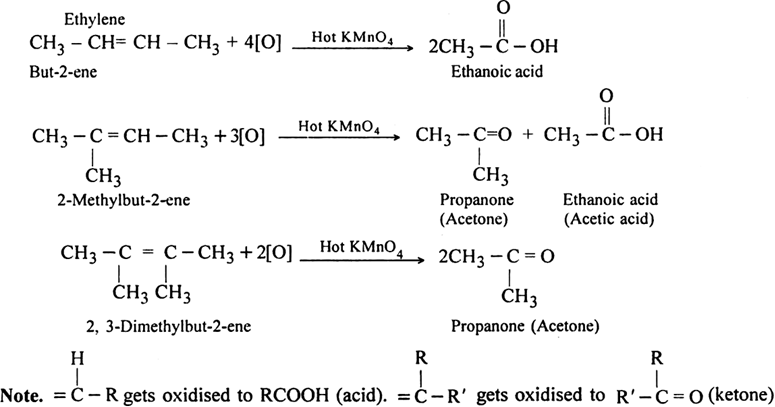
(iii) Using hydrogen peroxide (H2O2). Alkenes react with hydrogen peroxide to form diols.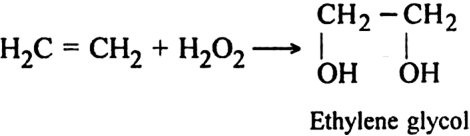
(iv) Using air in the presence of silver as a catalyst at 525-675 K. Ethylene forms ethylene oxide.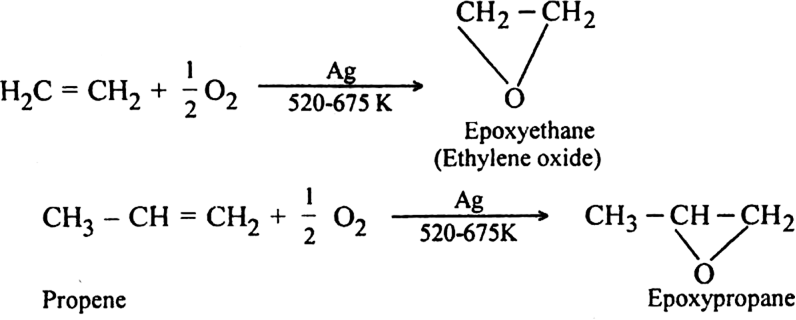
(v) Combustion. When burnt in air or oxygen, alkenes form carbon dioxide and water.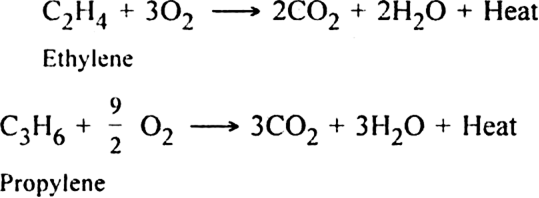
The reaction is highly exothermic in nature and the heat evolved is used for welding purposes.
Write structures of all the alkene which on hydrogenation give 2-methyl butane.
The basic skeleton of 2-methyl butane is:![]()
therefore the following alkenes or hydrogenation give 2-methyl butane.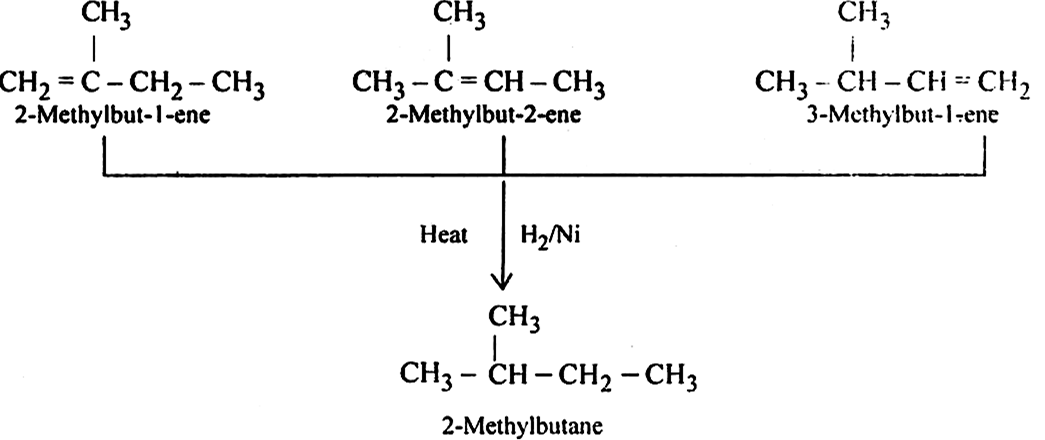
Give reduction of phenol ?
Reduction of phenol : Phenol is reduced to benzene by passing its vapours over-heated zinc dust.
Discuss in brief the polymerisation reactions of alkenes ?
Polymerisation: Polymerization is the process in which a large number of same or different molecules of unsaturated compounds combine to form a bigger molecule called polymer. The combining units are called monomers.
(i) Formation of polyethene: Ethylene polymerises under high pressure in the presence of traces of oxygen as a catalyst to give polythene or polyethylene.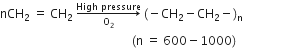
Polythene is mainly employed for the manufacture of toys, bottles, plastic pipes, polythene bags, etc.
(ii) Formation of Teflon. It is obtained by the polymerisation of tetrafluoroethylene.
It is highly resistant to heat and electricity and is used for insulating electric wires. Due to its chemical inertness, it is used for coating the inner surfaces of the non-sticking cooking pans.
(iii) Formation of polyvinyl chloride (PVC). When vinyl chloride is heated in an inert solvent in the presence of benzoyl peroxide catalyst, polyvinylchloride is formed.
It is used in making rain-coats, hand bags, table clothes, plastic dolls etc.
Enlist three uses of ethylene.
Ethylene is used:
(i) for ripening citrus fruits,
(ii) as an anaesthetic,
(iii) for making ethylene glycol which is used as a coolant for air-craft engines and as antifreeze for motor cars.
What are alkynes?
Alkynes are further classified as terminal and non-terminal according to as the triple bond is present at the end of the chain or in between the chain.
The hydrogen atom attached to the terminal carbon is known as acetylenic hydrogen and the anion formed by removing acetylenic hydrogen is called acetylide or alky nide ion.
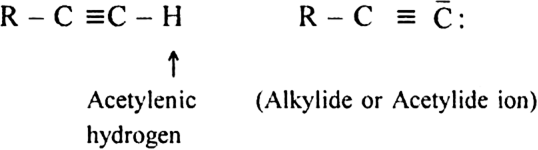
Naming of alkynes:
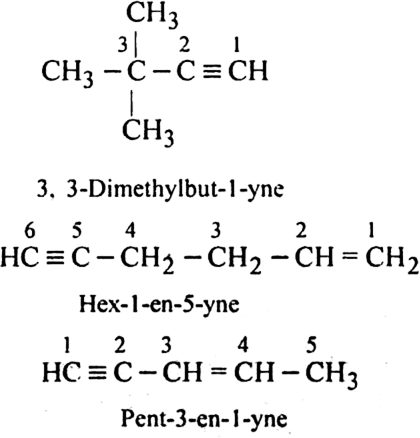
For the following compounds, write structural formulas and IUPAC names for all possible isomers having the number of double or triple bond as indicated:
(b) Isomers of C5H8 having one triple bond are:
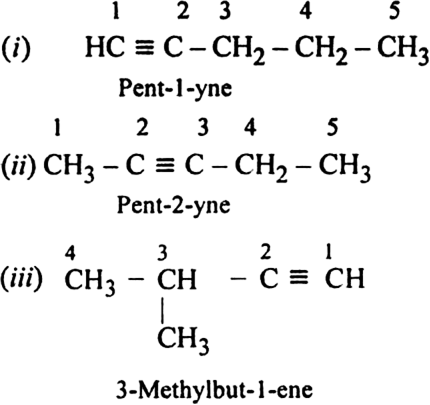
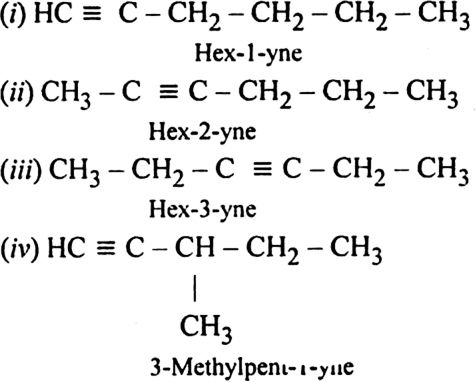
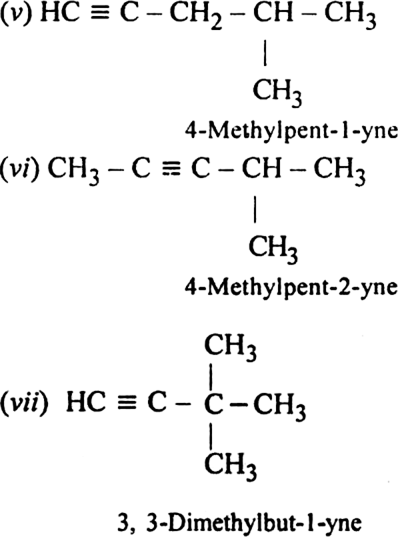
Write structures of different isomers corresponding to the 5th member of alkyne series. Also, write IUPAC names of all the isomers ?
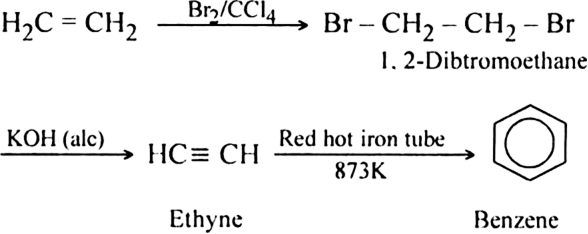
How is acetylene obtained from:
(i) Ethylene dibromide?
(ii) Tetrabromoethane ?
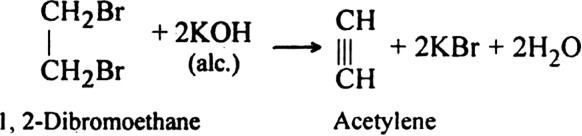
(ii) From tetrabromo ethane. Acetylene is prepared by heating tetrabromo ethane with zinc dust.
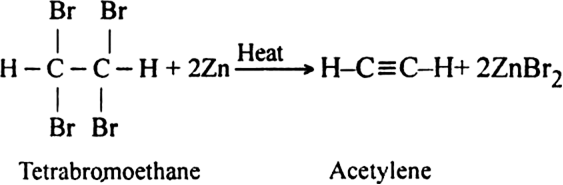
Discuss the preparation of higher alkynes.
(i) Acetylene is treated with sodamide in liquid ammonia, sodium acetylide is produced.
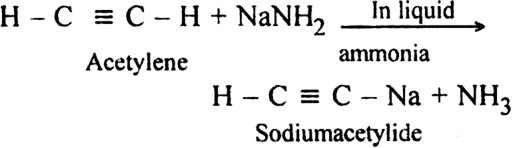
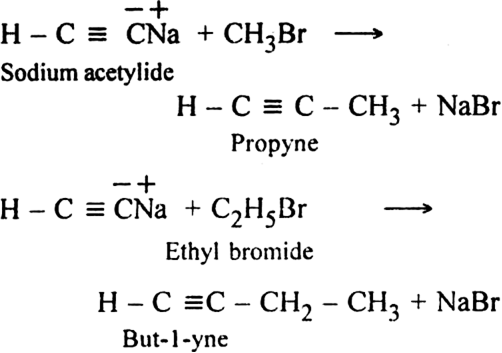
(ii) Sodium acetylide is then treated with proper alkyl halide, higher members of alkynes are produced.
Why do alkynes undergo electrophilic addition reactions ?

The addition product further reacts with E-Nu (attacking reagent) to form addition product i.e. alkane derivative.
Mechanism:
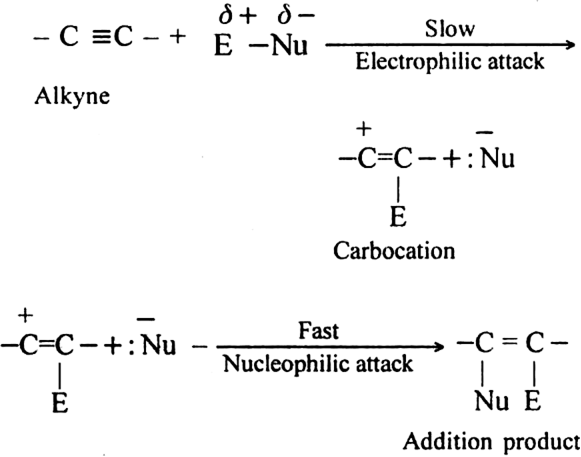
The electrophilic addition gets repeated and alkene derivative changes to alkane derivative.
Alkynes are less reactive towards electrophilic addition reactions than alkenes. Explain.
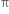 -electrons are more tightly held by the nuclei of carbon atoms in alkyne than in alkene. As a result, the
-electrons are more tightly held by the nuclei of carbon atoms in alkyne than in alkene. As a result, the 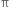 electrons are less easily available for combination with the electrophiles. Hence alkynes are less reactive than alkenes.
electrons are less easily available for combination with the electrophiles. Hence alkynes are less reactive than alkenes.Give two reactions of electrophilic addition on acetylene along with their mechanism.
Mechanism. It involves electrophilic addition and consists of the following steps:
Step II. The electrophilic addition is repeated again when the final compound gets formed.
(ii) The addition of hydrogen halides. The hydrogen halides (HCl, HBr or HI) add-on triple bond to give rise to haloalkene or haloalkane.
Mechanism. It involves electrophilic addition and consists of the following steps:
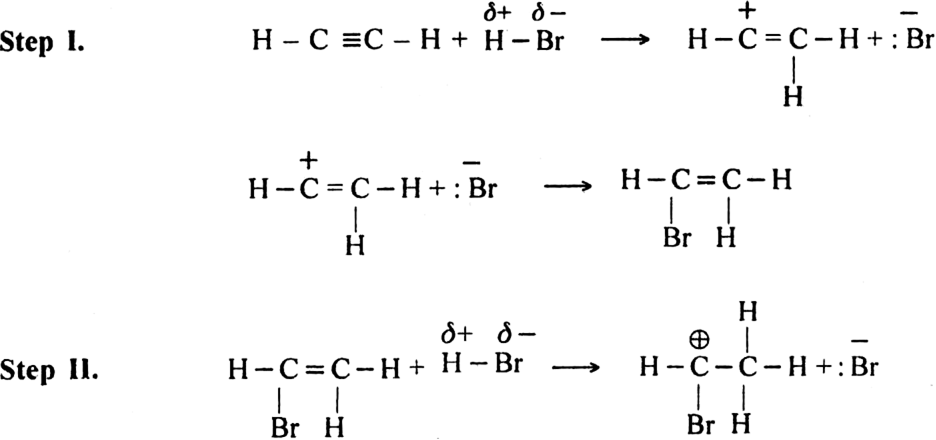
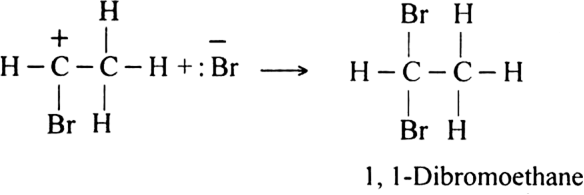
Give two reactions of electrophilic addition on acetylene along with their mechanism.
(i) The addition of halogen: Chlorine and bromine add on to acetylene to form addition product. ![]()
Mechanism: It involves electrophilic addition and consists of the following steps: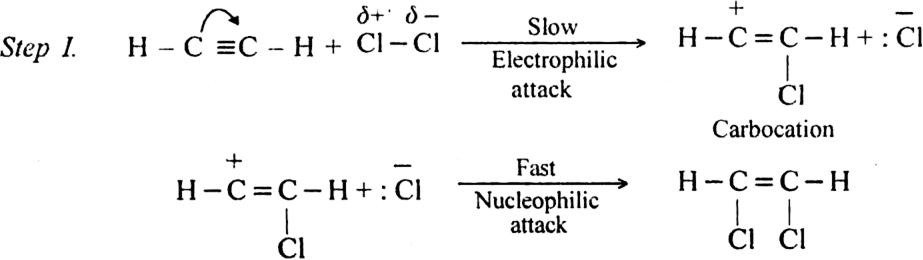
Step II. The electrophilic addition is repeated again when the final compound gets formed.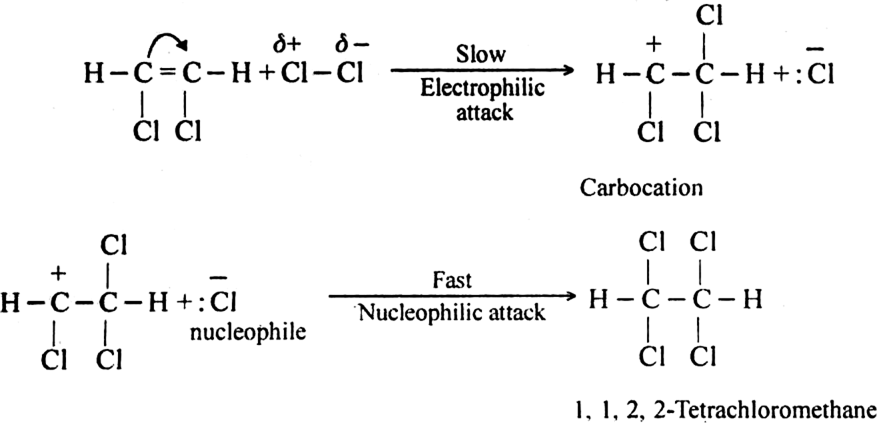
(ii) The addition of hydrogen halides. The hydrogen halides (HCl, HBr or HI) add-on triple bond to give rise to haloalkene or haloalkane.![]()
Mechanism: It involves electrophilic addition and consists of the following steps: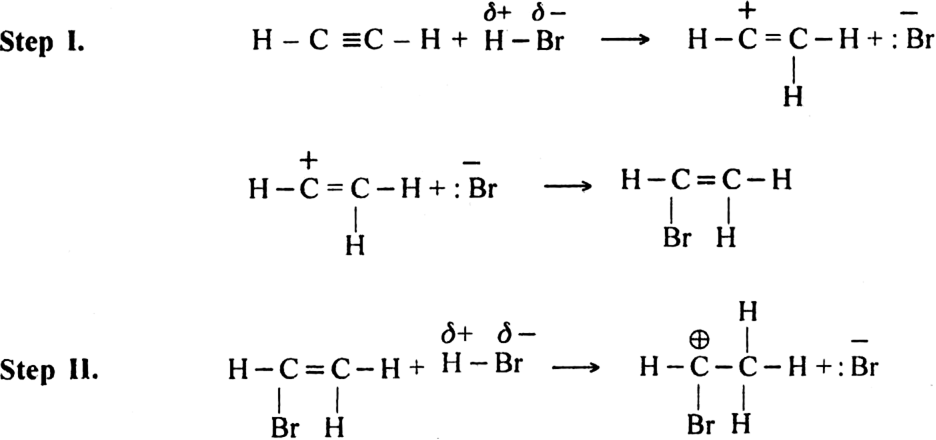
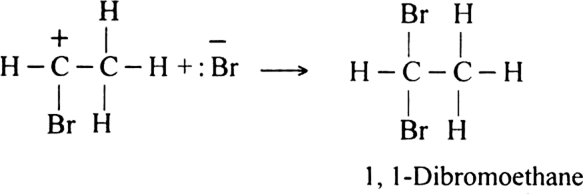
How do alkynes react with hypochlorous acids? Discuss its mechanism.
Or
Both hypochlorous acid HOCl (Cl2 | H2O) and hypobromous acid HOBr (Br2 | H2O) react with alkynes in two steps.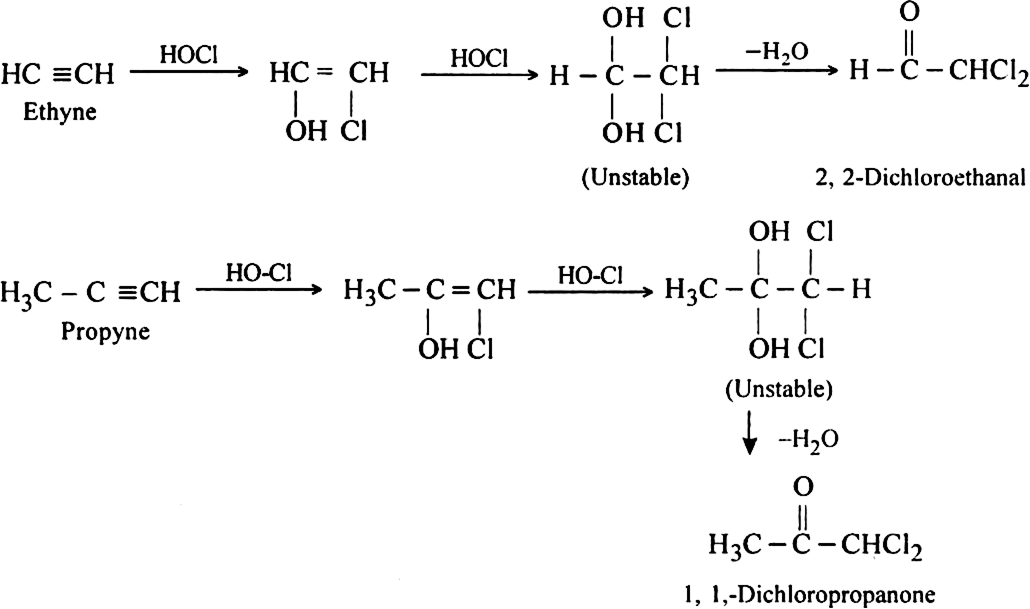
Mechanism: It is an example of electrophilic addition and proceeds in two steps: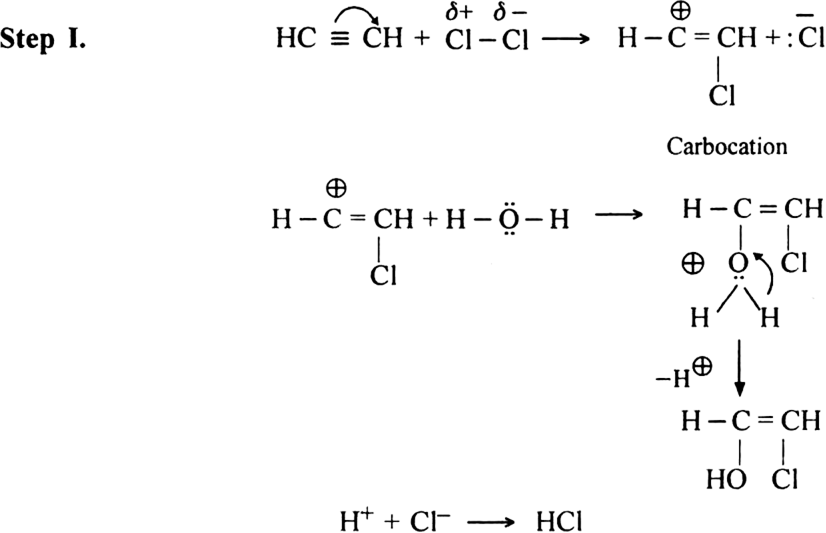
Step II. The electrophilic addition is repeated again when the final compound gets formed.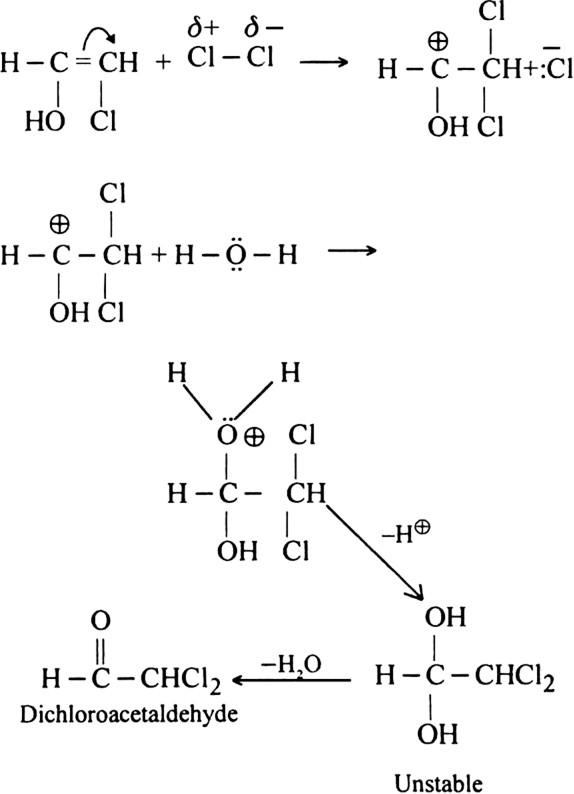
How does acetylene react with:
(i) Gaseous chlorine
(ii) Liquid bromine?
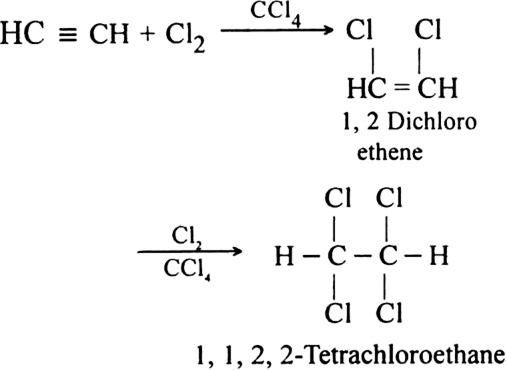
(ii) With liquid bromine: Acetylene reacts with liquid bromine to form tetrabromo derivative.
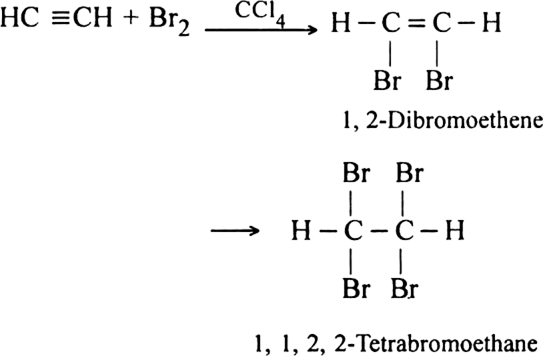
The red colour of bromine is discharged indicating the presence of unsaturation in the molecule.
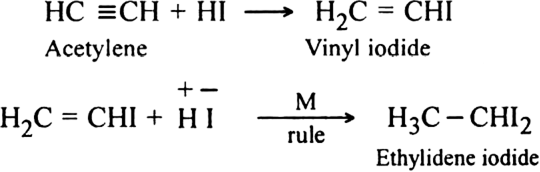
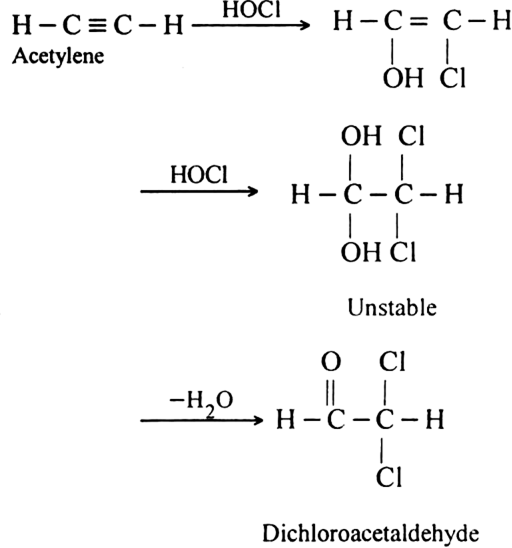
Starting from acetylene how will you obtain:
(i) Ethylidene iodide
(ii) Dichloro acetaldehyde
(iii) Ethylene?
(ii) Dichloro acetaldehyde. Acetylene reacts with hypochlorous acid to form dichloro acetaldehyde
.
(iii) Ethylene. Acetylene reacts with hydrogen in the presence of Lindlar’s catalyst (Pd poisoned with CaCO3) to form ethylene.
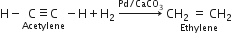
Give a brief account of the nucleophilic addition in alkynes.
Or
What is the role of Hg2+ ions in the nucleophilic addition reaction of alkynes?
 -cloud.
-cloud.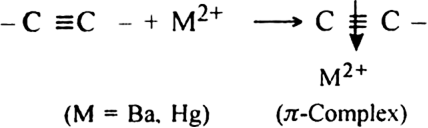
As a result, the electron density in the triple bond gets considerably reduced and therefore, the nucleophilic attack is facilitated.
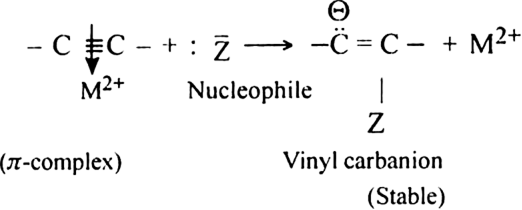
In other words,
 -complex formation is responsible for the nucleophilic addition reaction of alkynes. For example, alkynes undergo following nucleophilic addition reactions:
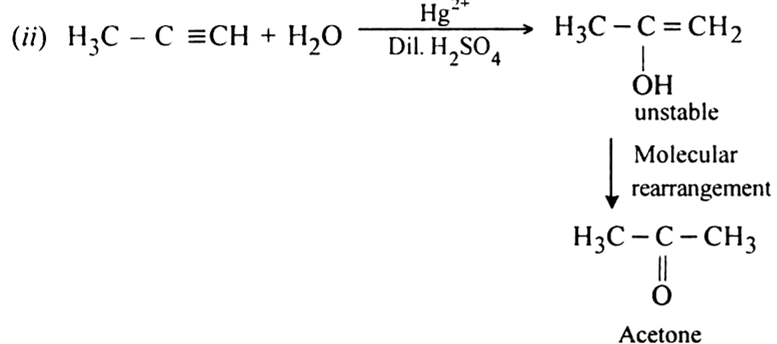
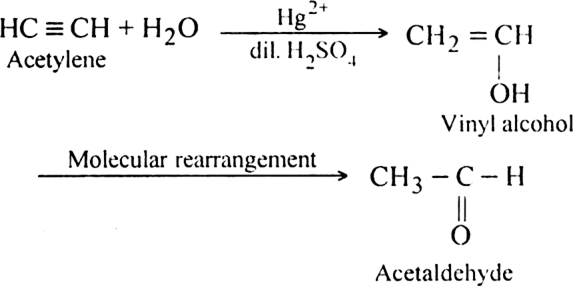
-complex formation is responsible for the nucleophilic addition reaction of alkynes. For example, alkynes undergo following nucleophilic addition reactions:(i) The addition of water in the presence of dilute H2SO4 and HgSO4.
(ii) The addition of HCN in the presence of Ba(CN)2.
(iii) The addition of acetic acid in the presence of Hg2+ ion.
How does acetylene react with:
(i) Water in the presence of dilute H2SO4 and HgSO4
(ii) HCN in the presence of barium cyanide?
(ii) The addition of hydrogen cyanide: Acetylene reacts with hydrogen cyanide in the presence of barium cyanide to form vinyl cyanide.

Explain the catalytic hydrogenation of alkynes.
Catalytic hydrogenation of alkynes: It involves two types of hydrogenation.
(i) Complete hydrogenation: When vapours of alkynes are passed over the surface of the catalyst like Ni, Pt or Pd at 473 K, hydrogenation takes place to form alkenes and then alkanes. For example,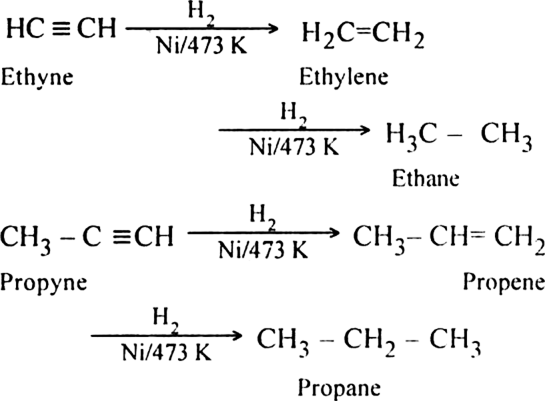
(ii) Controlled hydrogenation. When vapours of alkynes are passed over the heated Lindlar’s catalyst (Pd/CaCO3) partially poisoned by lead acetate or nickel boride catalyst known as P-2), controlled hydrogenation takes place only up to alkene stage.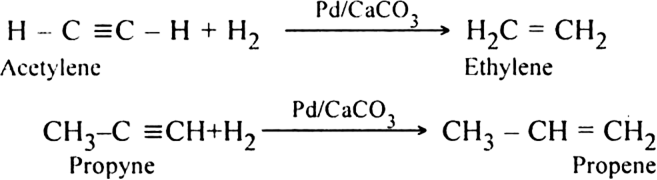
Starting from Ethyne, how will you prepare (No mechanism):
(i) Ethanol (ii) Vinyl chloride (iii) Methyl vinyl ether (iv) Vinyl acetate?
(i) Ethanol: It is obtained by the following steps of reaction: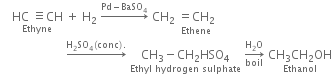
(ii) Vinyl chloride: Ethyne reacts with one mole of HCl to form vinyl chloride.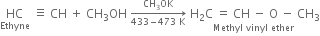
(iii) Methyl vinyl ether: When ethyne is passed into methyl alcohol at 433-473 K in the presence of small amount of potassium methoxide and under pressure, methyl vinyl ether is formed.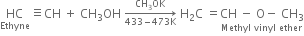
(iv) Vinyl acetate: Acetic acid adds on a molecule of ethyne in the presence of mercury salt to form vinyl acetate. 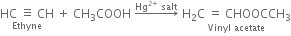
How will you convert:
(i) Propyne into acetone
(ii) Ethyne into propyne?
(i) Propyne into acetone: This can be done by the addiction of water in the presence of dilute sulphuric acid and Hg2+ ions.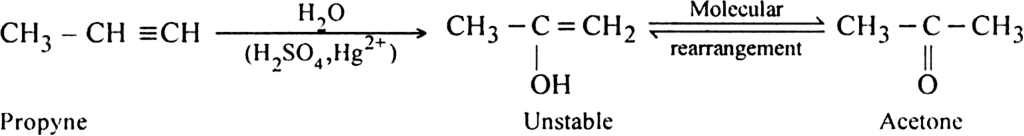
(ii) Ethyne into propyne: This can be done by the following steps: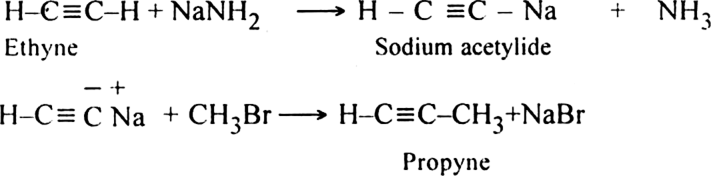
Discuss in brief the oxidation reactions of alkynes.
Different products are formed depending upon the nature of oxidising agent:
(i) Using cold dilute KMnO4 (Baeyer’s reagent): Alkynes are oxidised by cold dilute KMnO4 to form 1, 2-diketones or carboxylic acids.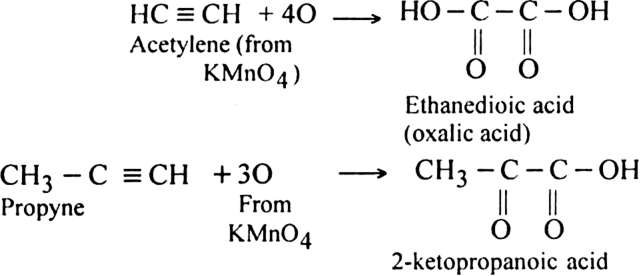
The pink colour of KMnO4 gets discharged. Therefore, this reaction is used as a test for unsaturation in the molecule.
(ii) Using hot KMnO4: When alkynes are treated with hot KMnO4, a triple bond is completely broken forming carboxylic acids and carbon dioxide depending upon the structure of alkyne.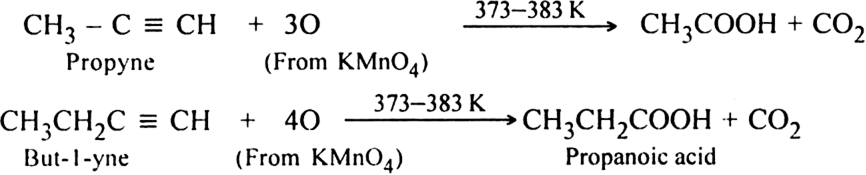
(iii) Oxidation with ozone. The reaction of alkynes with ozone followed by reduction with zinc and water results in the formation of diketones. e.g.
(iv) Combustion: Acetylene and higher alkynes burn when heated with air or oxygen to form CO2 and H2O. The reaction is highly exothermic in nature.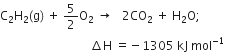
The heat evolved during the reaction is employed as an oxyacetylene flame for welding purposes.
Write briefly the polymerisation reactions of alkynes.
Alkynes acetylene is passed through a solution of cuprous chloride and ammonium chloride in hydrochloric acid, two molecules are joined together to form vinyl acetylene.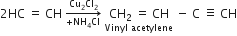
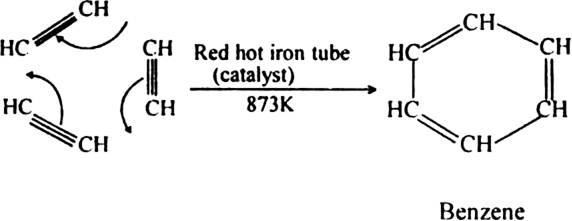
(ii) When vapours of acetylene are passed through red hot iron tube at 773 K, three molecules polymerise to form benzene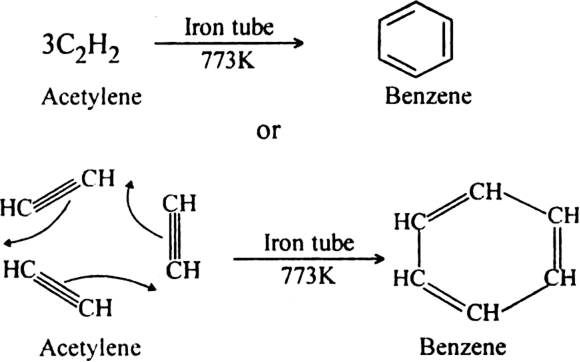
(iii) When vapours of acetylene polymerise in the presence of nickel cyanide and under high pressure, four molecules are joined together to form cyclooctatetraene. 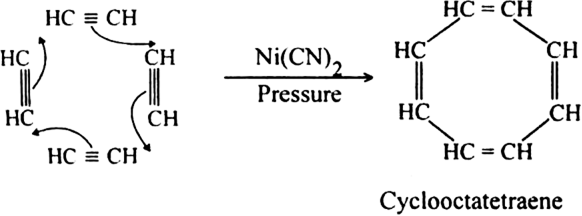
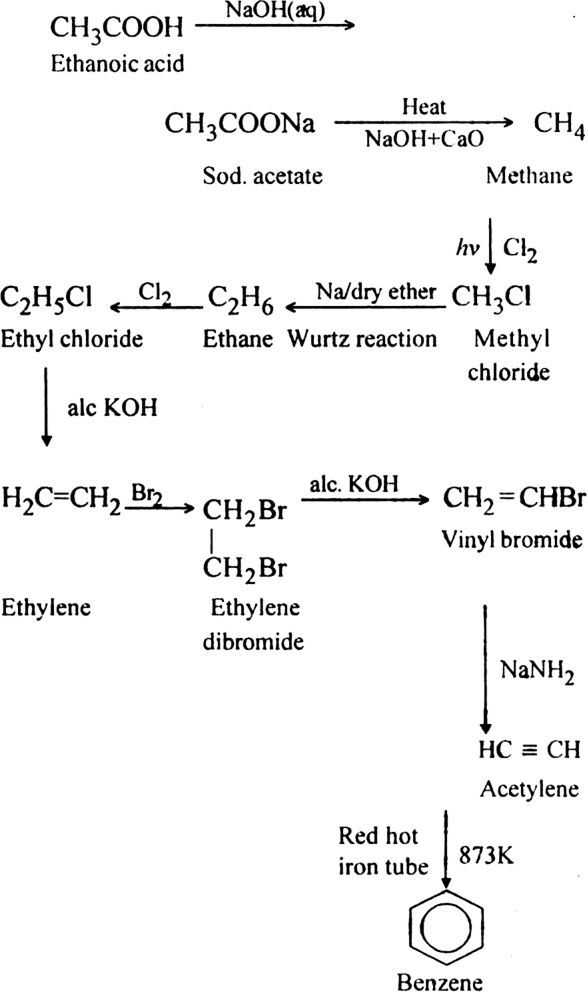
How will you convert ethanoic acid into benzene ?
Discuss the acidic character of alkynes.
Alkynes with a triple bond in the terminal position (– C ≡ C – H) are very weakly acidic in nature. They do not turn blue litmus red but give hydrogen with metals like sodium.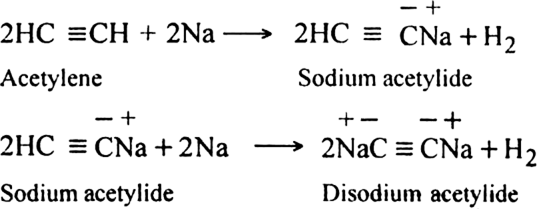
Explanation: The hydrogen atom attached to the triply bonded carbon atom ( C – C ≡ C – H) is called acetylenic hydrogen. The acidic character of alkyne having acetylenic hydrogen (HC ≡C –H, CH3 – C ≡ C – H, etc.) is explained by hybridization phenomenon. The carbon atom in ≡ C–H bond beings hybridised (50%, s-character) is quite electronegative in nature. It can readily accept the electron pair from the C-H bond. Consequently, the H+ ion can be easily released and this accounts for the weakly acidic character of alkynes.
Write three reactions to show the acidity of alkynes.
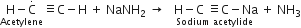
(ii) Action with ammonical silver nitrate (Tollen's reagent): When acetylene is passed through ammonical silver nitrate, a white precipitate of silver acetylide is produced.
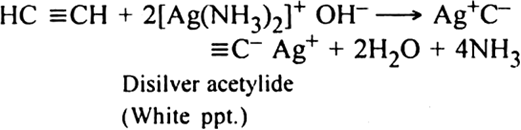
(iii) Action with ammonical cuprous chloride: When acetylene is passed through ammonical cuprous chloride, a red precipitate of cuprous acetylide is produced.
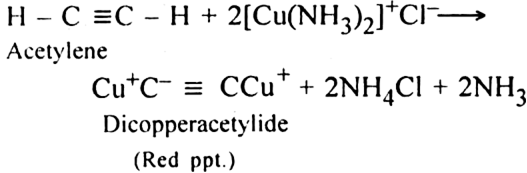
All these reactions indicate that acetylenic hydrogen atoms are acidic in nature.
Alkynes are acidic in nature, whereas alkenes and alkanes are not. Explain.
The acidic character of alkynes can be explained by the concept of orbital electronegativity. The triple bonded carbon atoms of alkynes use two sp hybridised orbitals in the formation of σ bonds.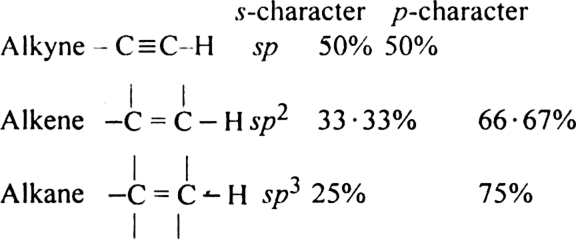
The carbon atoms of alkynes have greater s-character as compared to the double bonded carbon atoms of alkenes and singly bonded carbon atoms of an alkane. Now s-orbital penetrates more towards the nucleus, the electrons in it are more tightly held. The carbon atoms of the ( C ≡ C) triple bond become more electronegative than sp2 and sp3 hybridised carbon atom. The more electronegative carbon atom thus attracts the shared pair of electrons more strongly. As a result, a proton is easily knocked out from – C ≡ C – H as compared to ![]() and
and ![]() , hence acidic character. CH3 – C ≡ – CH3(But-2-yne) is not acidic because it does not contain any hydrogen attached to triple bonded carbon atom.
, hence acidic character. CH3 – C ≡ – CH3(But-2-yne) is not acidic because it does not contain any hydrogen attached to triple bonded carbon atom.
Arrange benzene, n-hexane and ethyne in decreasing order of acidic behaviour. Also give reason for his behaviour.
The decreasing order of acidic strength:
Hydrocarbon: : Ethyne> Benzene > n-Hexane
Hybridization of C-atoms : (sp) (sp2) (sp3)
The acidic character is linked with the percentage of s-character. Greater the s-character, more is the electronegativity of the carbon atom and more will be the acidic character.
Give simple chemical test for the distinction of:
(i) Propyne and propane
(ii) But-1-yne and But-2-yne.
(i) Propyne and propane: Add a cold, dilute alkaline KMnO4 solution to each compound and shake it well. Propyne will decolourise the pink colour of KMnO4 while propane does not.
(iii) But-1-yne and But-2-yne: Add few drops of an ammoniacal solution of silver nitrate to each compound and warm. But-1-yne will give white precipitate while but-2-yne does not.
How will you separate acetylene from a mixture of acetylene and ethylene?
Bubble the gaseous mixture through an ammoniacal solution of silver nitrate (Tollen's reagent) when acetylene products white precipitate of disilver acetylide. Ethylene will remain in the gaseous state and can be separated. 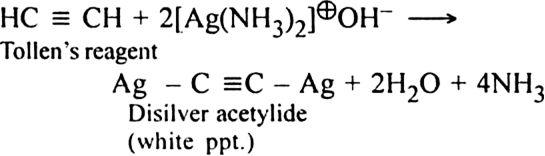
The precipitate is separated, treated immediately with dilute HNO3 to get back acetylene in pure form.![]()
You are given samples of ethane, ethene and ethyne in three different containers. How will you distinguish them?
(i) A small portion of each given sample is treated separately, with Br2/CCl4. The sample which does not decolourise is ethane. Because ethene and ethyne both form product with Bromine.
(ii) The remaining two samples which decolourise bromine are treated separately with ammoniacal solution of silver nitrate. The sample which produces white precipitate is ethyne. Thus, the third sample is clearly ethene.
What are aromatic hydrocarbons or arenes?
Hydrocarbons and their alkyl, alkenyl and alkyl derivatives which contain one or more benzene ring either fused or isolated in their molecules are called aromatic hydrocarbons. They are also called arenes. Since such compounds resemble benzene in almost all of their properties, they are also called benzenoid compounds. A few members of the family are,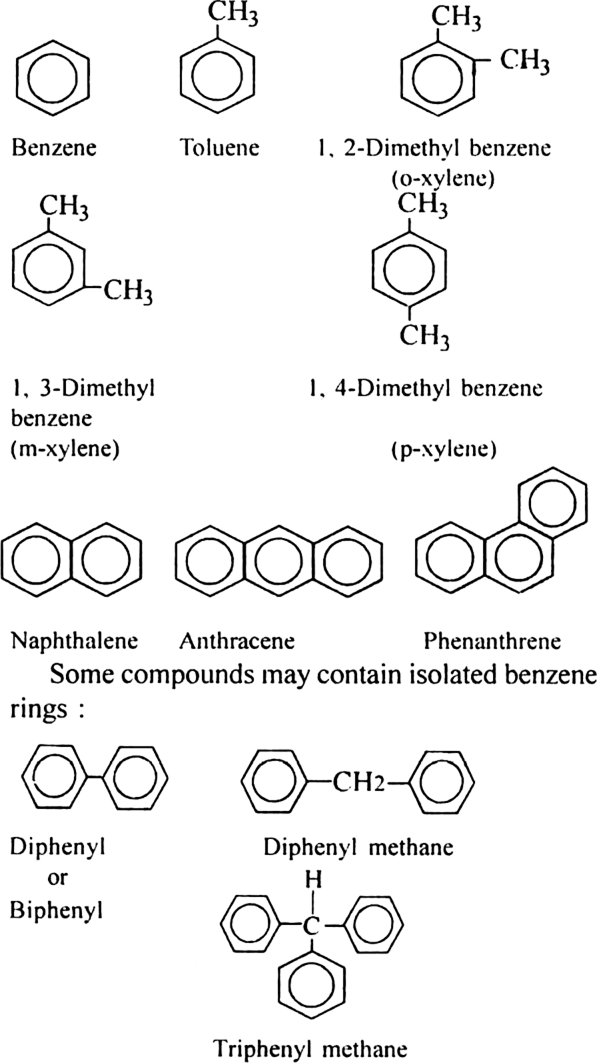
Explain isomerism in arenes.
Arenes show position isomerism in their molecules. The isomers differ with respect to the relative positions of the alkyl substituents. In disubstituted derivates (-CH3 group on the substituent), three isomers are possible.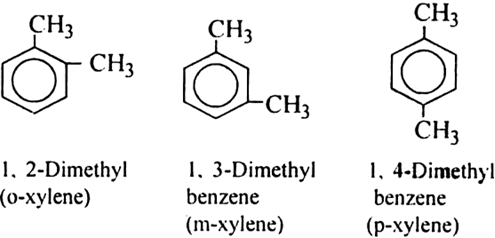
Similarly, three position isomers of trimethyl benzene are: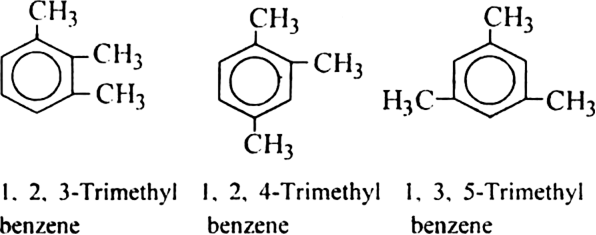
In the case of bicyclic arenes such as naphthalene, even monosubstituted compounds show position isomerism. For example. 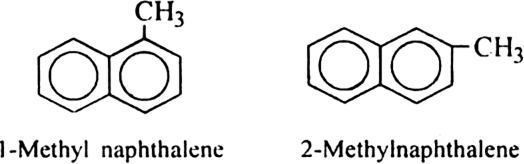
Discuss the structure of benzene with special reference to Kekule structure.
The molecular formula of benzene is C6H6 indicating a high degree of unsaturation. As a result, benzene is expected to be highly reactive compound like alkenes or alkynes. Therefore benzene should:
(i) decolourise aqueous potassium permanganate.
(ii) add water in the presence of acids.
(iii) decolourise bromine water.
But actually, benzene does not undergo such reactions. This implies that benzene is different from alkenes and alkynes. Moreover, being extraordinarily stable, it behaves more like alkanes than an alkene or alkynes. It prefers to undergo substitution reactions rather than addition.
Since benzene on catalytic hydrogenation forms cyclohexane (a ring compound), this suggests that the carbon skeleton of benzene should be a six-membered cyclic structure.
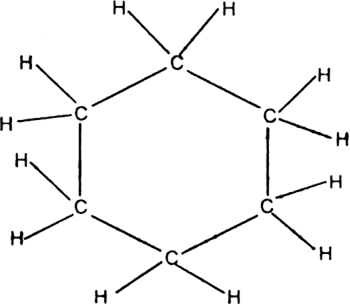
Kekule’s structure of benzene: Kekule proposed that benzene has a ring structure in which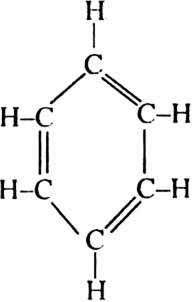
the six carbon atoms are arranged in the form of a regular hexagon and one hydrogen each is bonded to each carbon atom. Since carbon is tetravalent, he proposed that alternate single and double bonds are present between carbon atoms.
Objections to Kekule’s structure:
(i) Kekule’s structure allows the existence of two isomeric orthos-substituted dibromo benzene (I and NY).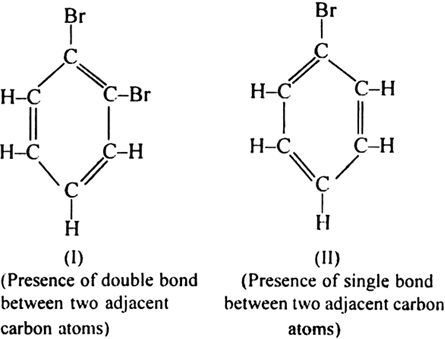
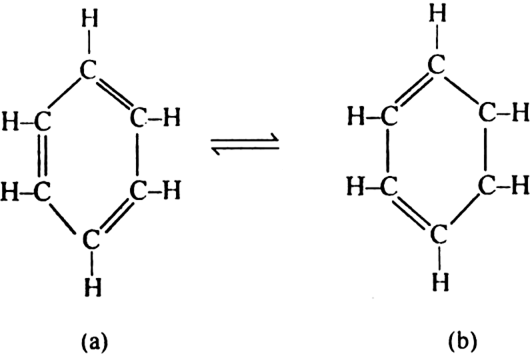
(ii) Kekule’s structure does not explain the extra ordinary stable nature of benzene molecule and its lack of reactivity towards addition reactions, resistance towards oxidation etc.
(iii) Equivalence of all the carbon-carbon bond lengths in benzene.
Discuss the molecular orbital structure of benzene (Delocalisation of  -electrons).
-electrons).

The unhybridized 2pz orbital on each carbon atom can overlap sidewise with the 2pzorbital of the two adjacent carbon atoms in two different ways as shown below giving rise to two sets of
 -bonds. Since 2pz orbital on any carbon atom can overlap sideways with the 2pz orbital on adjacent carbon atom on either side equally well, a continuous
-bonds. Since 2pz orbital on any carbon atom can overlap sideways with the 2pz orbital on adjacent carbon atom on either side equally well, a continuous  -molecular 3 orbitals will result which embraces all the six p-electrons as shown:
-molecular 3 orbitals will result which embraces all the six p-electrons as shown: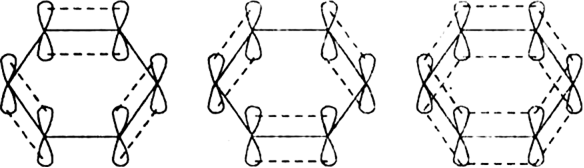
The net result is that there are two continuous rings-like electron clouds, one above and the other below the plane of atoms as shown. This delocalisation of π-electrons imparts unique stability to the benzene molecule.
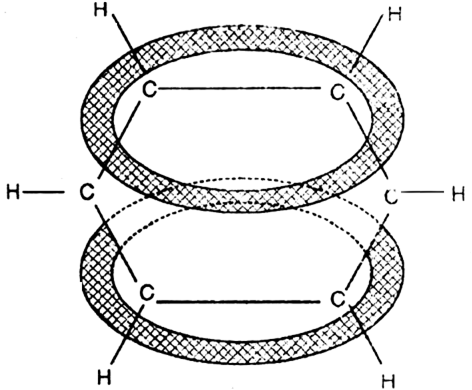
In benzene molecule all
What do you mean by delocalisation of  -electrons?
-electrons?
 -electrons are delocalised throughout the whole molecule. The delocalisation of
-electrons are delocalised throughout the whole molecule. The delocalisation of  -electrons to a greater space causes a decrease in energy and hence increases the stability of benzene molecule. Delocalisation of electrons is possible in coplanar molecules.
-electrons to a greater space causes a decrease in energy and hence increases the stability of benzene molecule. Delocalisation of electrons is possible in coplanar molecules.
Benzene ring has three double bonds in it but is still quite stable. Explain.
Or
Why is benzene extra-ordinary stable though it contains three double bonds?
 -electrons are delocalised throughout the whole molecule. This delocalisation of
-electrons are delocalised throughout the whole molecule. This delocalisation of  -electron clouds above and below the plane of benzene ring causes a decrease in energy and hence increases the stability of benzene molecule.
-electron clouds above and below the plane of benzene ring causes a decrease in energy and hence increases the stability of benzene molecule.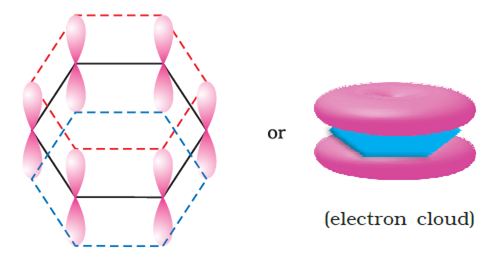
Why carbon-carbon distance in benzene is intermediate between carbon-carbon single and double bond?
Each carbon atom of benzene is sp2 hybridised. The unhybridised p-orbital of each carbon atom overlaps to a small but equal extent with the unhybridised p-orbitals of both the adjacent carbon atoms. This leads to the formation of molecular orbital embracing all the six carbon atoms. Due to the delocalisation of  –electron clouds above and below the plane of the benzene ring, all the carbon-carbon bonds acquire slight double bond character. Thus carbon-carbon bond length in benzene lies in between C–C and C=C bond length.
–electron clouds above and below the plane of the benzene ring, all the carbon-carbon bonds acquire slight double bond character. Thus carbon-carbon bond length in benzene lies in between C–C and C=C bond length.
Standard C–C bond length = 154 pm
Standard C=C bond length = 134 pm
But C–C bond length in benzene = 139 pm.
What is resonance? Discuss the resonance in benzene. What is the effect of resonance ?
A resonance may be defined as a phenomenon in which a single compound is supposed to be existing as a hybrid of two or more compounds differing in the distribution of electrons and not of atoms. These different structures of the molecule are known contributing structures or resonating structures or canonical forms. The actual structure that is intermediate between all the contributing structures is called resonance hybrid. Different contributing structures are written by putting a double head arrow ( ↔ ) between them.
Resonance in benzene: Benzene ring has three double bonds in it and is expected to be quite reactive. But benzene is extremely stable. The stability of benzene is explained in terms of resonance. Benzene molecule is a resonance hybrid of the following two main contributing structures: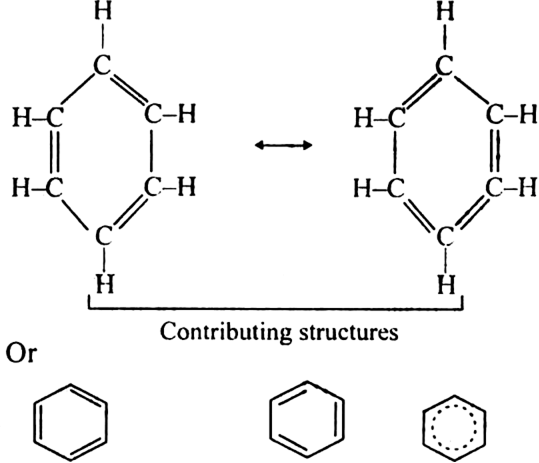
Due to resonance in benzene, the carbon-carbon bonds in benzene acquire an intermediate character of carbon-carbon single and double bonds. As a result, each carbon-carbon bond length in benzene is 139 pm which lies between standard C – C bond length 154 pm and C=C bond length 134 pm.
Effect of resonance: Due to resonance, the 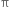 -electron charge in benzene is distributed over a greater area. The density of the charge decreases. As a result, the energy of resonance hybrid also decreases or its stability increases.
-electron charge in benzene is distributed over a greater area. The density of the charge decreases. As a result, the energy of resonance hybrid also decreases or its stability increases.
What is resonance energy?
It is defined as the difference in energy between the energy of the most stable of the contributing structures and energy of the actual molecule (resonance hybrid).
Resonance Energy = Energy of most stable contributing structure - Energy of resonance hybrid.
The resonance energy of benzene is approximately 150 kJ mol-1 .
What are the characteristics of a compound to be aromatic?
Or
What do you mean by aromaticity?
Or
What are the necessary conditions for any system to be aromatic?
The compounds possessing aromatic character show the following characteristics:
(i) The compounds must be cyclic in nature and have flat planar structure.
(ii) Their molecular formulae suggest these compounds are highly unsaturated due to the presence of one or more double bonds in the ring but they must behave as saturated compounds.
(iii) They must resist addition reaction and take part in the electrophilic substitution reactions.
(iv) The molecules have delocalised  electron cloud above and below the plane of the ring.
electron cloud above and below the plane of the ring.
(v) An essential criterion for the aromatic character is that the compound must obey Huckel’s rule. According to this rule, a cyclic compound will behave as aromatic compound if it contains (4n + 2) electrons, where n may be 0, 1, 2, 3 etc. Huckel’s rule can be applied successfully to polycyclic compounds, annulenes and also other non-benzenoid compounds. For example;
electrons, where n may be 0, 1, 2, 3 etc. Huckel’s rule can be applied successfully to polycyclic compounds, annulenes and also other non-benzenoid compounds. For example;
(a) Monocyclic systems: Some monocyclic systems having π-electrons (obey Huckel’s rule) possess aromatic character.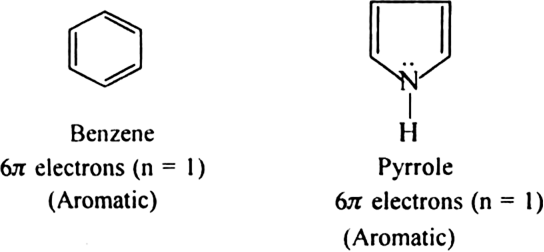
(b) Fused ring systems: The polynuclear hydrocarbons such as naphthalene, anthracene and phenanthrene are also aromatic according to Huckel’s rule.
Aromatic ions: Some cyclic ions also exhibit aromatic character. For example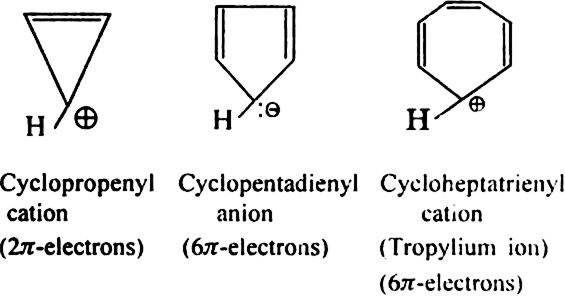
The following compounds are not aromatic: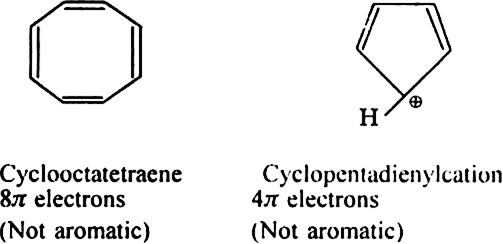
Cycloheptatriene although obeys Huckel’s rule yet it is not aromatic as it is not planar and can not show resonance.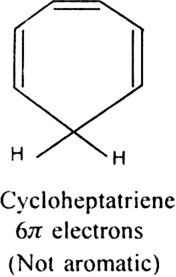
Write a short note on annulenes.
Annulenes are monocyclic conjugated polyenes which contain an even number of carbon atoms in their molecules. These are represented by the general formula (-CH = CH-)n where n = 2, 3, 4.....
Aromatic nature in annulenes: According to Huckel’s rule, annulenes containing (4n + 2) electrons and having a coplanar cyclic carbon skeleton should be aromatic in nature. For example,
(i) [4] Annulene. It is non-aromatic because it does not contain 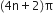 electrons.
electrons.
(ii) [6] Annulene. It is aromatic because it contains 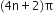 electrons.
electrons.
(iii) [8] Annulene. It is non-aromatic because it does not contain 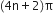 electrons.
electrons.
Explain why the following systems are not aromatic?![]()
Since the above structure contains sp3 hybridised carbon, therefore the system is not planar. Though it can six
 electrons yet the system is not fully conjugated. In other words, all the six
electrons yet the system is not fully conjugated. In other words, all the six  -electrons do not form a single cyclic electron cloud which surrounds all the atoms of the ring. Hence it is not an aromatic compound.
-electrons do not form a single cyclic electron cloud which surrounds all the atoms of the ring. Hence it is not an aromatic compound. 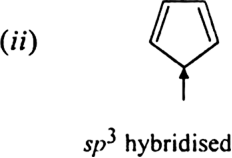
The system is not planar due to the presence of sp3 hybridised carbon. Also,it contains four electrons, indicating that the system is not aromatic i.e. the system does not obey (4n + 2)n-electrons.
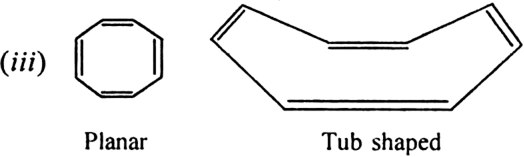
The system is not planar but has a tub-like a shape. It is, therefore, a non-planar system having
 . Hence the molecule is not aromatic since it does not obey (4n+2)
. Hence the molecule is not aromatic since it does not obey (4n+2)  .
.Give the various methods used for the preparation of arenes.
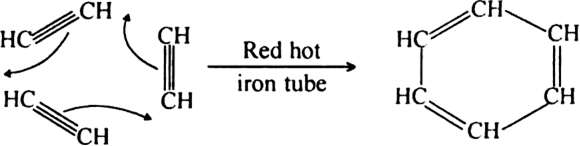
(ii) From chlorobenzene: By reducing chlorobenzene with Ni-Al alloy/NaOH.

(iii) From sodium benzoate: By heating sodium benzoate with soda lime (Laboratory method).
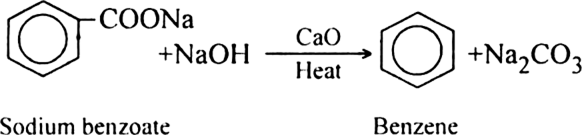
(iv) From phenol: By distilling phenol with zinc dust.
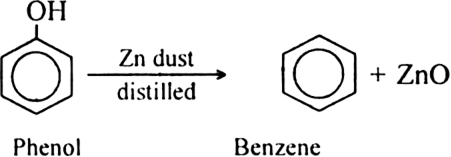
(v) From benzene sulphonic acid: By heating benzene sulphonic acid with super heated steam.
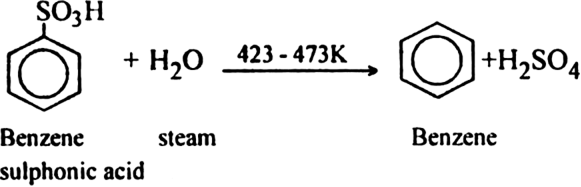
(vi) From benzene diazonium chloride: By treating benzene diazonium chloride with hypophosphorous acid (H3PO2) in the presence of Cu+ ions.
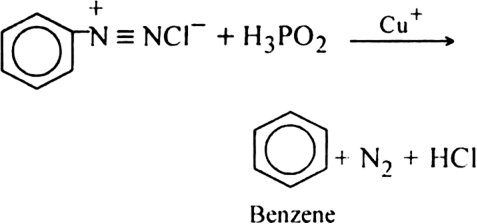
(vii) By Wurtz-Fittig reaction: By treating halorene and haloalkane with sodium metal in the presence of dry ether.
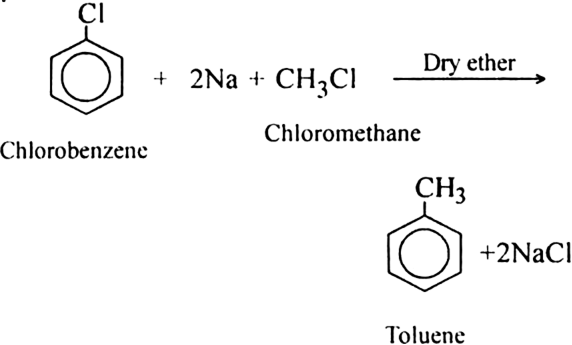
How would you convert the following compounds to benzene:
(i) Benzoic acid
(ii) Benzene diazonium chloride?
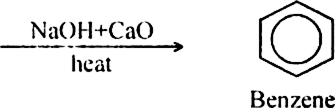
(ii) Benzenediazonium chloride to benzene:
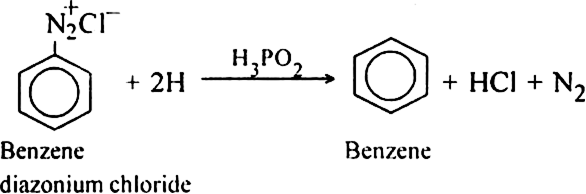
Discuss the halogenation reactions of arenes.
Halogenation: Benzene reacts with chlorine or bromine in the presence of ferric or aluminium halide at room temperature to form substituted product. 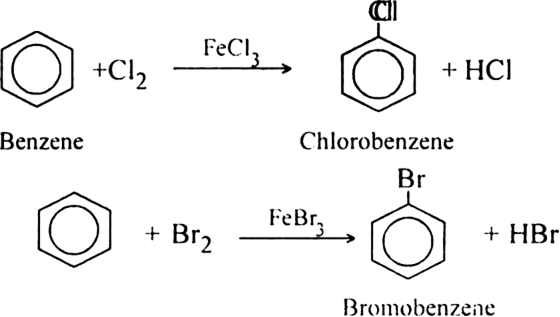
Iodination: Reaction of benzene with iodine is not possible because of its reversible nature i.e. HI formed reduces the iodo derivative back to the starting hydrocarbon.
However, the forward reaction is carried out by heating benzene with iodine in the presence of iodic acid (HIO3) which oxidises HI formed to iodine.
Similarly, toluene also undergoes halogenation under similar conditions giving a mixture of ortho and para disubstituted products.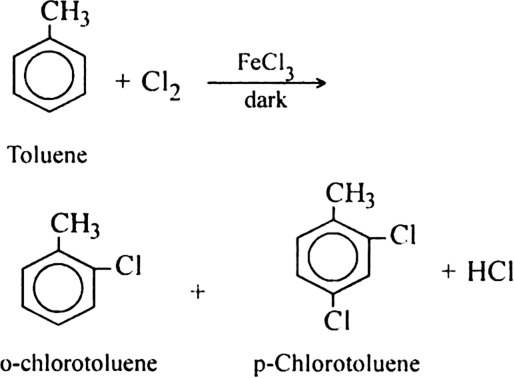
Discuss:
(i) nitration and (ii) sulphonation of benzene.
(ii) Sulphonation: When benzene is heated with concentrated H2SO4 or fuming sulphuric acid at 373K, benzene sulphonic acid is produced.
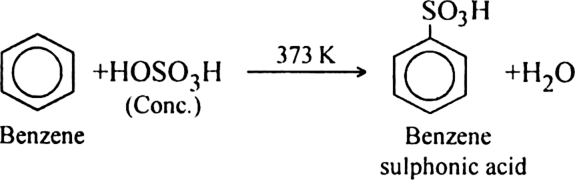
Write a short note on Friedel Craft’s reaction ?
Friedel Craft's reaction are two types:
i)Friedel-Crafts alkylation:
ii)Friedel-Crafts acylation reaction:
i) In Friedel Craft's benzene is treated with an alkyl halide in the presence of anhydrous aluminium chloride, alkylbenzene is formed.
(i) Using alkyl halide (alkylation reaction):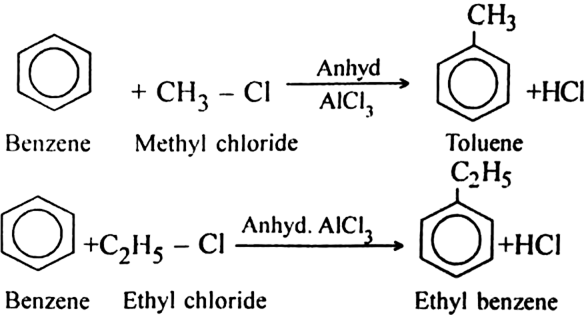
(ii) Friedel-Crafts acylation reaction:
The reaction of benzene with an acyl halide or acid anhydride in the presence of Lewis acids (AlCl3) yields acyl benzene.
Discuss the important addition reactions of benzene.

(ii) The addition of halogens. Benzene reacts with chlorine or bromine in the presence of sunlight and absence of halogen carrier to form benzene hexachloride or benzene hexabromide.
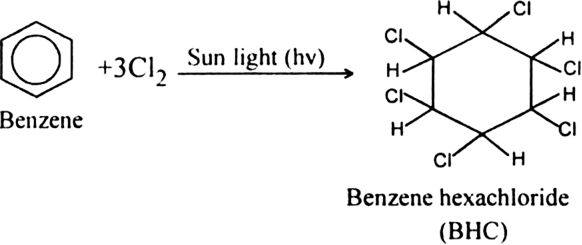
BHC is used as an insecticide. It is sold under the name Gammexane or lindane.
(iii) The addition of ozone. Benzene reacts with ozone to form benzene tri ozonide.
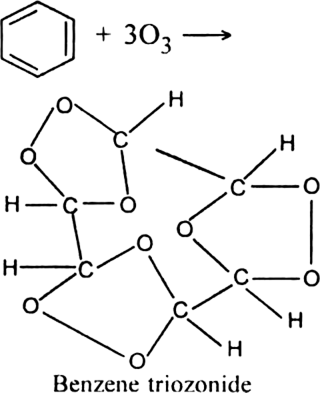
Discuss the oxidation reactions of arenes.
(i) Combustion: When burnt in oxygen, benzene and other hydrocarbons burn with a smoky flame and produce carbon dioxide and water.
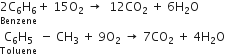
(ii) Oxidation of benzene: When a mixture of air and vapours of benzene is passed over vanadium pentoxide (V2O5) at 773 K, maleic anhydride is produced.
(iii) Oxidation of the side chain.
(a) With weak oxidising agent. Toluene is oxidised to benzaldehyde by using chromyl chloride (CrO2Cl2) or acidic manganese dioxide.
(b) With normal oxidising agent. On oxidation with a strong oxidising agent such as hot KMnO4, concentrated HNO3, acidified KMnO4 or acidified dichromate etc., the entire side chain, regardless of length, is oxidised to - COOH group.
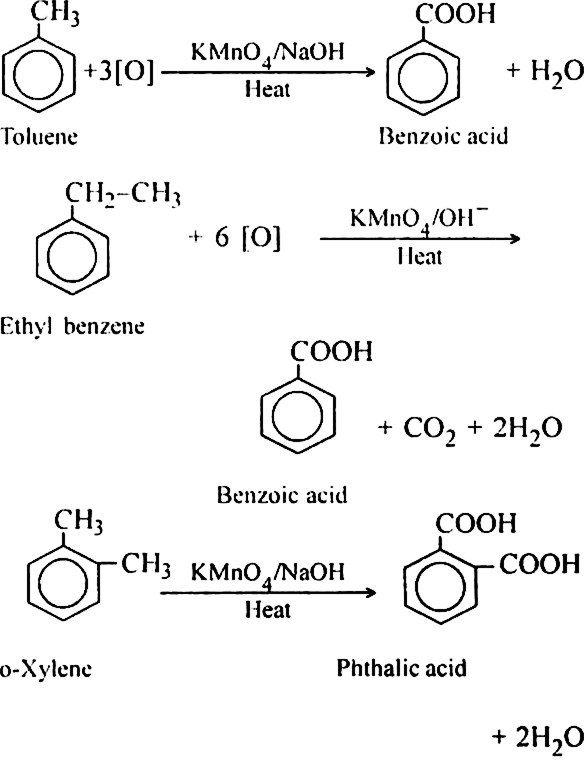
Write down the products of ozonolysis of 1, 2-dimethylbenzene (o-xylene). How does the result support Kekule structure for benzene?
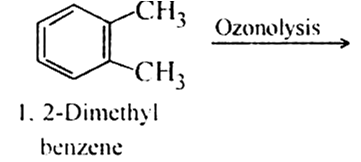
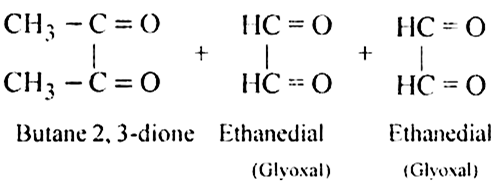
All these products can be possible only in case, there are three double bonds in the ring in the alternate positions. Thus products of ozonolysis support Kekule structure.
What happens when:
Benzene is treated with acetic anhydride in the presence of anhydrous AlCl3.
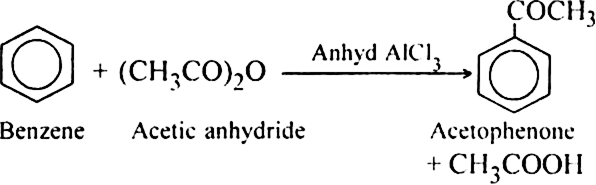
What happens when:
Toluene is heated with a mixture of concentrated nitric acid and concentrated sulphuric acid at 303 K.
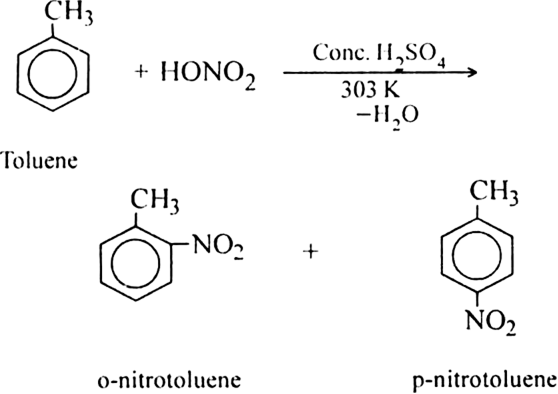
What happens when:
Toluene is heated with concentrated sulphuric acid.
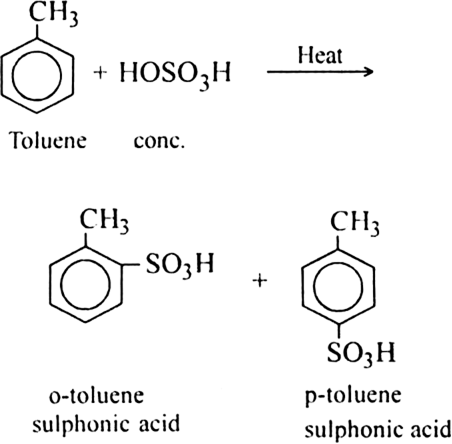
What happens when:
p-xylene is heated with alkaline KMnO4.
What happens when:
Benzene is treated with n-propyl chloride in the presence of anhydrous AlCl3.
Discuss the mechanism of electrophilic substitution reactions of benzene?
Typical reactions of benzene ring namely halogenation, nitration, sulphonation, and Friedel-Craft reaction are electrophilic substitutions.
Benzene ring serves as a source of electrons (nucleophile) due to the presence of electron cloud. The electrophilic reagent will attack the aromatic nucleus and the hydrogen atom of benzene is displaced by the electrophilic reagent. A substituted product is formed via intermediate carbonium ion formation.
Such reactions which are initiated by the electrophiles are called Electrophilic substitution reactions. The product formed is known as substituted product.
Let us consider a reaction of the attacking reagent E - N on the benzene in the presence of a catalyst. Substituted product is formed. 
Mechanism. It involves the following steps:
(i) Generation of an electrophile (E+): The function of the catalyst is to produce an electrophile from the attacking reagent E - N.
(ii) Formation of intermediate carbocation: The electrophile (E+) then is attacked by the n electrons of the benzene ring to form an intermediate carbocation. It is resonance stabilised.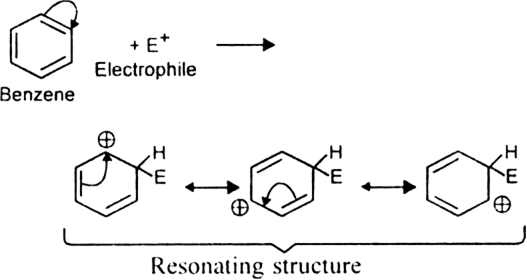
The resonance hybrid structure of the above resonance forms can be represented as
(iii) Catalyst-regenerating step.The base (Catalyst:N-) then removes the hydrogen ion from the intermediate carbocation and aromatization takes place forming the final product.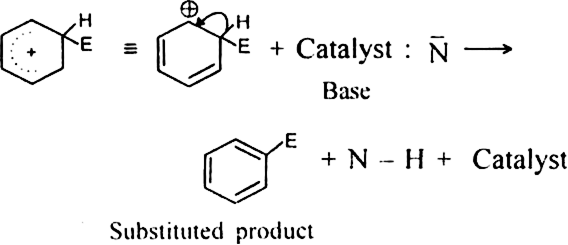
Discuss the mechanism of chlorination of benzene.
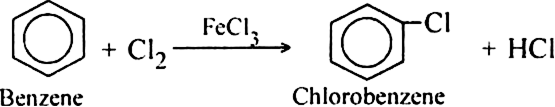
Different steps involved in the mechanism are:
(i) Generation of an electrophile (Cl+): The catalyst Lewis acid (FeCl3) helps in the generation of an electrophile (Cl+) by causing polarisation of the chlorine molecule.
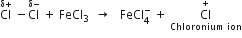
(ii) Formation of the carbocation: The electrophile (Cl+) attacks the benzene ring to form an intermediate carbocation which is resonance stabilised. It is a slow step.
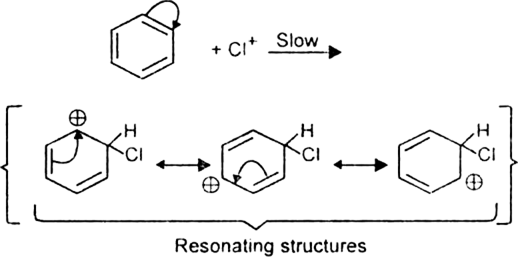
Resonance hybrid of the above resonating structures can be represented as,
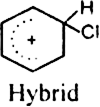
(iii) Proton transfer from the carbocation to form chlorobenzene.
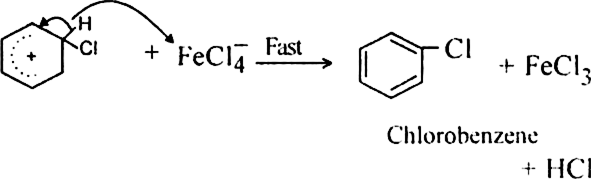
How is benzene converted to nitrobenzene? Give mechanism.

Mechanism: Different steps involved in the mechanism are:
(i) Generation of nitronium ion (electrophile):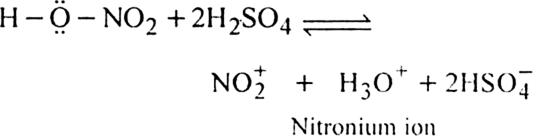
(ii) Formation of carbocation: The electrophile 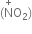 attacks the benzene ring to form an intermediate carbocation which is resonance stabilised. It is a slow step.
attacks the benzene ring to form an intermediate carbocation which is resonance stabilised. It is a slow step.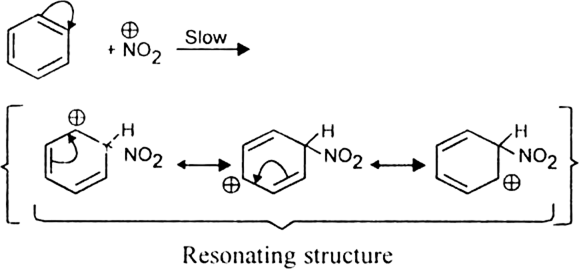
The resonance hybrid of the above resonating structures can be represented as,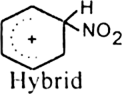
(iii) Proton transfer from the carbocation to yield nitrobenzene.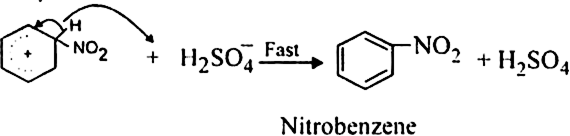
How benzene is converted into benzene sulphonic acid? Give its mechanism.
Or
Explain the mechanism of sulphonation of benzene.
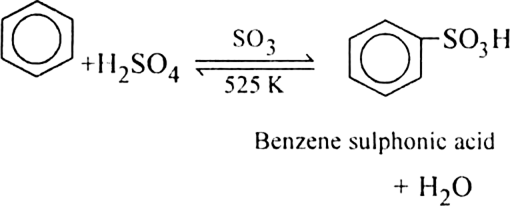
Mechanism: It involves the following steps:
(i) Generation of sulphur trioxide (electrophile). The attacking electrophile sulphur trioxide, SO3 (neutral but electron deficient) is present as such in oleum (H2SO4 + SO3) or can be formed by the dissociation of the sulphuric acid.
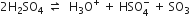
The sulphur atom in sulphur trioxide has the only sextet of electrons. Thus the molecule is electron deficient in nature.
(ii) Formation of the carbocation. The electrophile (SO3) attacks the benzene ring to form intermediate carbocation which is resonance stabilised. It is a slow step.
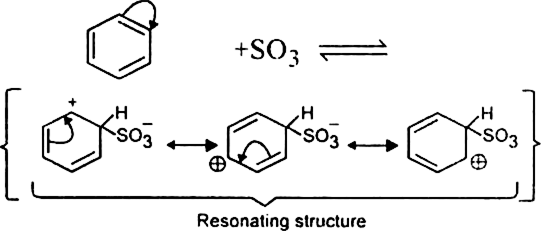
The resonance hybrid structure of the above resonating form can be represented as
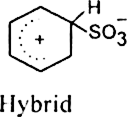
(iii) Transfer of proton from the carbocation to form benzene sulphonate ion.
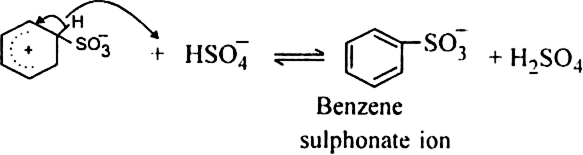
(iv) The reaction between benzene sulphonate ion and hydronium ion to form benzene sulphonic acid.
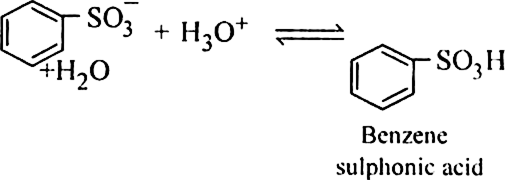
Discuss the mechanism of Friedel Craft’s reaction.
Or
Discuss the mechanism of Friedel Craft’s alkylation of benzene. Give the mechanism of reaction of benzene within ![]() the presence of AlCl3.
the presence of AlCl3.
Or
How is benzene converted into aceto-phenone? Name the reaction and discuss the mechanism explaining each step.
Or
Give the mechanism of acylation of benzene.
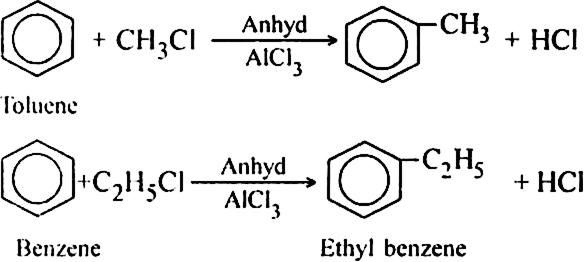
Mechanism. It involves the following steps:
(i) Generation of electrophile:
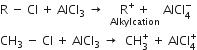
(ii) Formation of carbocation:
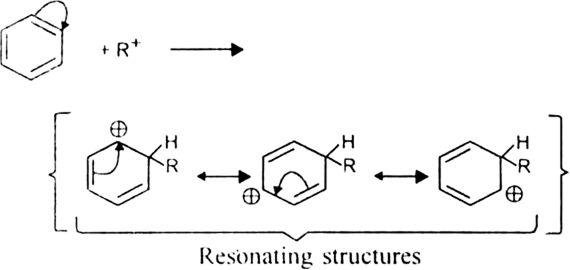
The resonance hybrid structure of the above resonating form can be represented as:

(iii) Transfer of proton from the carbocation to form alkyl benzene:

[B] Friedel-Craft acylation reaction. In acylation of benzene, the hydrogen attached to the carbon atom of the ring gets replaced by the acyl group (RCO-). For example,
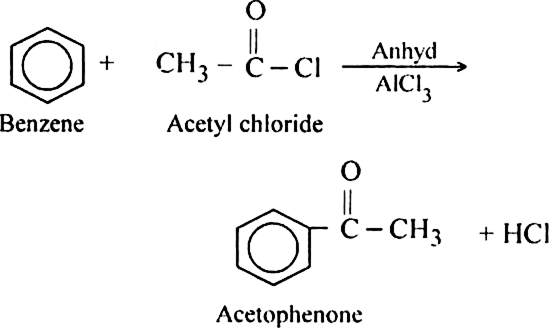
Mechanism: It involves following steps:
(i) Generation of electrophile.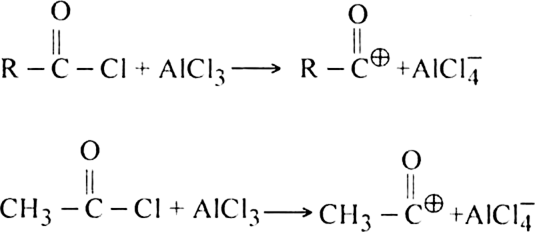
(ii) Formation of the carbocation.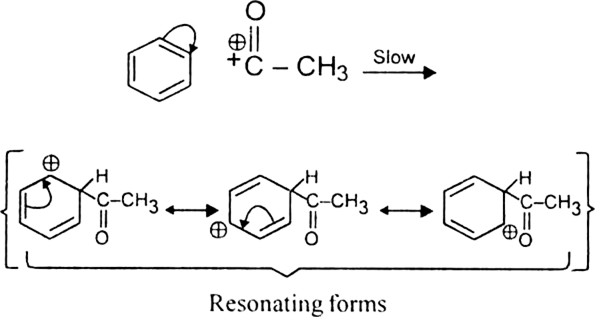
The resonance hybrid structure of the above resonating forms can be represented as: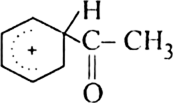
(iii) Proton transfer from the carbocation to form the final product. 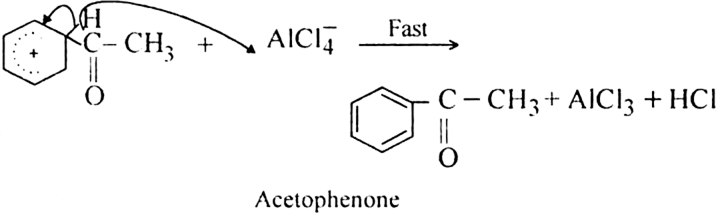
Why does benzene undergo electrophilic substitution reactions easily and nucleophilic substitutions with difficulty?
Benzene is a rich source of an electron because of the presence of electron cloud containing  -electrons above and below the plane of the ring. As a result, it attracts the electrophiles (electron-deficient species) towards it and repels nucleophiles (electron-rich species). Hence benzene undergoes electrophilic substitution reactions easily.
-electrons above and below the plane of the ring. As a result, it attracts the electrophiles (electron-deficient species) towards it and repels nucleophiles (electron-rich species). Hence benzene undergoes electrophilic substitution reactions easily.
Discuss the directive influence of substituents on disubstitution in benzene.
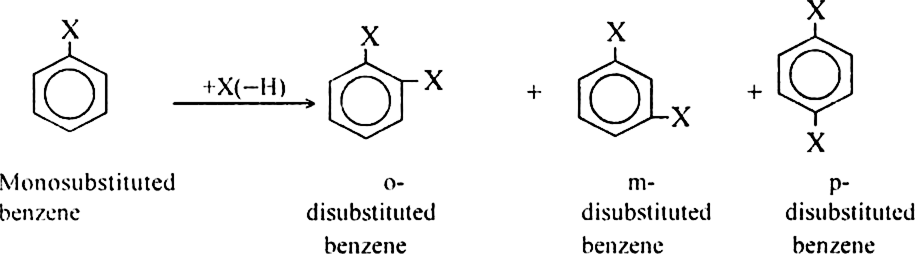
The position to be occupied by the second entering group depends on upon the nature of the group already present. This ability of a group already present in the benzene ring to direct the incoming group to a particular position is called the directive influence of groups. The groups have been classified into two types:
(i) Ortho and para directing groups: Such groups are directing the incoming group to attack at the ortho and para positions. For example,
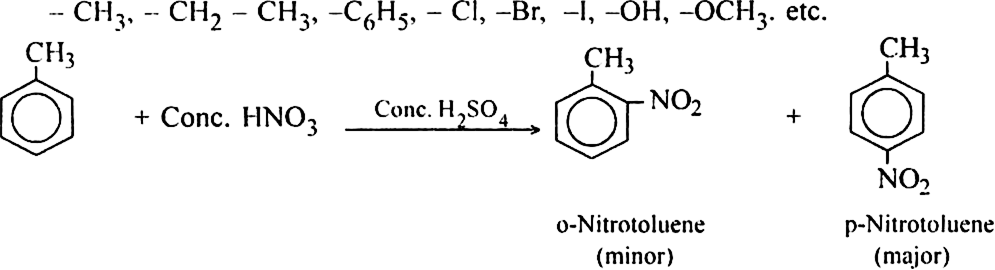
In general, all electron donating groups are o, p-directing.
(ii) Meta directing groups. Such groups direct the incoming group to attack at the meta position. For example -NO2, - CN, -CHO, -COR, -COOH, -SO3H etc.
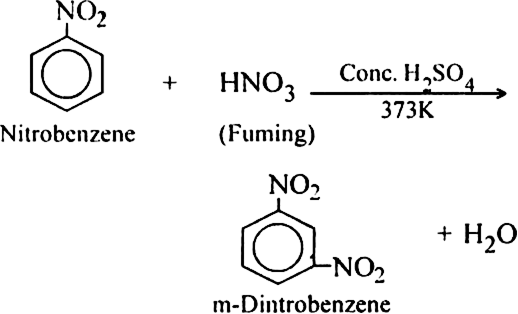
In general, all electron withdrawing groups are m-directing.
How will you convert benzene into:
(i) p-Nitrobromobenzene
(ii) m-Nitrochlorobenzene
(iii) p-Nitrotoluene
(iv) Acetophenone?
These conversions are based on the fact that:
(a) Chlorine is ortho and para directing in nature.
(b) Nitro group is meta directing in nature.
(i) Benzene into p-nitro bromobenzene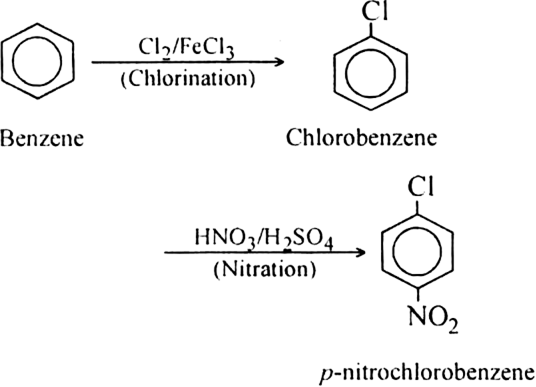
(ii) Benzene into m-nitro chlorobenzene.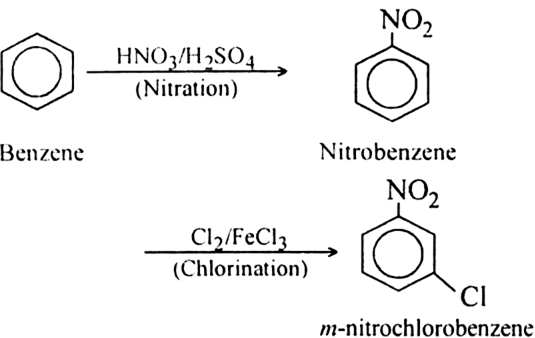
(iii) Benzene into p-nitrotoluene.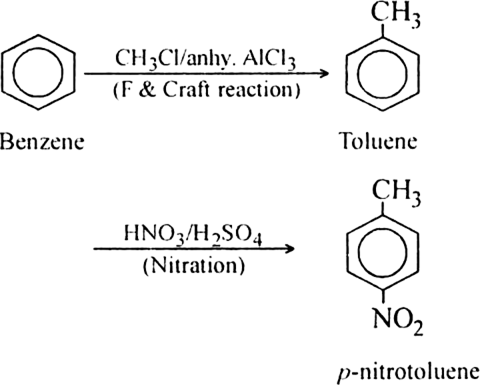
(iv) Benzene into acetophenone.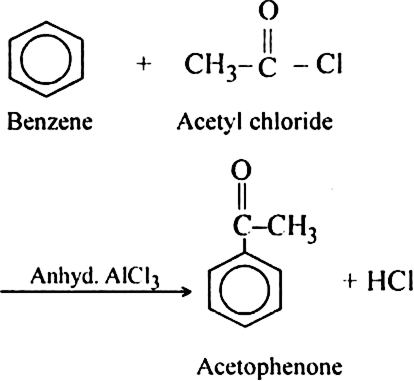
Out of benzene, m-dinitrobenzene and toluene which will undergo nitration most easily and why?
 (nitronium ion) on the ring. Since CH3 group has a +inductive effect, it activates the ring and electrophilic substitution readily takes place. On the other hand, it is most difficult in m-dinitrobenzene because of deactivating nature of nitro groups.
(nitronium ion) on the ring. Since CH3 group has a +inductive effect, it activates the ring and electrophilic substitution readily takes place. On the other hand, it is most difficult in m-dinitrobenzene because of deactivating nature of nitro groups.Thus ease of nitration decreases in the order Toulene> Benzene> m-Dinitrobenzene.
How will you explain the directive influence of alkyl group (in the case of toluene)?
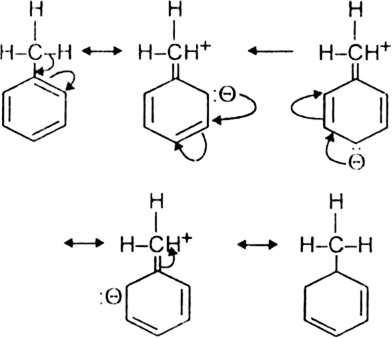
Thus –CH3 group is ortho and para directing.
How will you explain the directive influence of –NH2 group (in the case of aniline)?
The lone pair present on the nitrogen atom is in resonance or conjugation with the  electrons present in the ring. As a result, the ortho and para positions in the ring become the points of high electron density or negatively charged. The new entering group (electrophile) will prefer to attack at the ortho and para position rather than the meta position.
electrons present in the ring. As a result, the ortho and para positions in the ring become the points of high electron density or negatively charged. The new entering group (electrophile) will prefer to attack at the ortho and para position rather than the meta position. 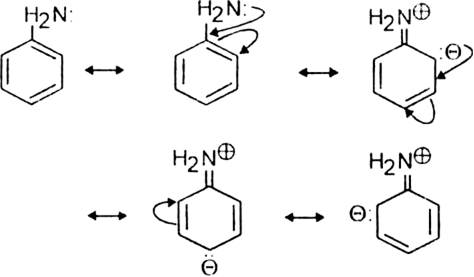
How will you explain the directive influence of -CHO group?
This can be explained by considering benzaldehyde in which -CHO group is an electron withdrawing group. The resonation structures of the molecules are:
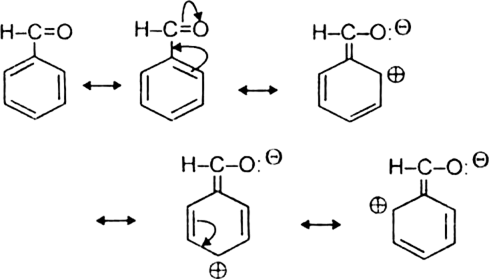
The meta positions become comparatively the centre of high electron density. Hence -CHO group directs the incoming group (electrophile) to attack at the m-position.
How will you explain the directive influence of halogens?
Halogens (Cl, Br, l) contain three pairs of electrons and thus release electrons to the aromatic ring through resonance. But due to its electrons withdrawing (-Inductive effect) nature, it also intensifies the positive charge on carbocation. Thus, inductive effect and resonance effect work in the opposite direction and the result of two opposing effects is electron withdrawal. That is why halogens deactivate the aromatic ring for electrophilic substitution. Consider chloro-benzene.+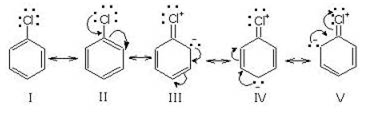
Thus chlorine atom deactivates the ring due to -I effect and directs the incoming electrophile to attack at the ortho and para position due to resonance effect.
Arrange the following set of compounds in order of their decreasing relative reactivity with an electrophile E+:
(a) Chlorobenzene; 2, 4-dinitrochlorobenzene, p- nitro chlorobenzene
(a) 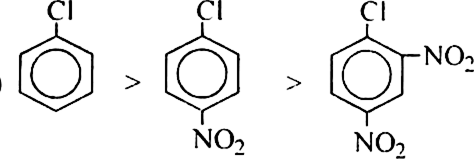
Nitro (NO2) group is a deactivating group. Its presence on the benzene ring will deactivate it towards electrophile attack since electrophile seeks a centre of high electron density. Thus, more the number of nitro groups present, lesser will be the reactivity of the compounds towards electrophilic substitution.
(b) Here, CH3 group is electron donating but NO2 group is electron withdrawing. Therefore, the maximum electron density will be in toluene, followed by p-nitrotoluene followed by p-dinitrobenzene. Thus, the overall reactivity decrease in the order: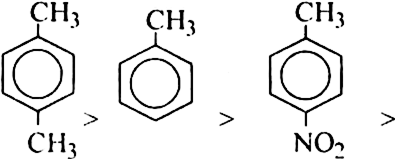
What are polynuclear hydrocarbons? Discuss the toxicity of polynuclear hydrocarbons.
(i) Fused polynuclear hydrocarbons: These are condensed aromatic hydrocarbons which contain more than one ring and have two carbons shared by two or more aromatic rings.
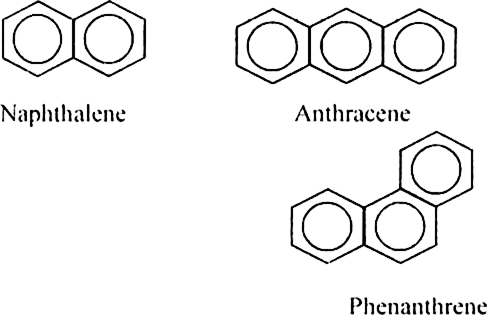
(ii) Isolated polynuclear hydrocarbons. In isolated polynuclear hydrocarbons, one or more benzene rings are linked to each other through one or more carbon atoms.
Toxicity of polynuclear hydrocarbons:
There are certain polynuclear hydrocarbons which are cancer producing (i.e. carcinogenic nature). Actually, these are the products of incomplete combustion of organic material such as coal, petroleum, tobacco etc. and become a major cause of human cancer. The following polynuclear hydrocarbons are cancer producing:
It is believed that when these polynuclear hydrocarbons enter the body of a human being, they are first converted into their oxides called epoxides and then into dihydroxy epoxides. The dihydroxy epoxides thus produced react with purine bases (such as guanine) present in DNA and RNA of the human cell, thereby causes mutation and ultimately leads to cancer. Also all over the world, steps are being taken to check the release of such toxic polynuclear hydrocarbons in the atmosphere.
What is liquified petroleum gas (L.P.G.)?
This is generally a mixture of propane and butane (n-butane as well as isobutane) compressed under pressure as a liquid and stored in iron cylinders. It is the major source of household fuel because the combustion of both propane and butane is almost complete without any unburnt carbon released to the atmosphere.
Discuss measures for the control of pollution problems with special reference to the use of C.N.G.
The engine of an automobile discharges a large number of gaseous substances into the atmosphere which includes carbon monoxide, carbon dioxide, water vapours, oxides of nitrogen (NOx), unburnt hydrocarbons and also certain compounds of lead if leaded petrol is used. Some of these compounds are highly poisonous such as compounds of lead. In order to check this, the automobiles are filled with catalytic converters to oxidise these harmful hydrocarbons to CO2, and H2O.
Now a days, use of C.N.G. (compressed natural gas), liquified petroleum gas (L.P.G.) and liquefied hydrogen gas (L.H.G.) have been tested in metropolitan cities. C.N.G. is mainly a mixture of methane and ethane and a small amount of propane. C.N.G. is normally found to be associated with crude petroleum. It is compressed and stored in cylinders. Its combustion is almost complete and no poisonous vapours are released in the environment. However, special types of engines have to be filled in the vehicles where C.N.G. is used. C.N.G. is quite successful in controlling air pollution.
What are petrochemicals? Discuss their importance.
Petrochemicals are the compounds which are derived either from petroleum or natural gas. Important petrochemicals are methane, ethane, propane, ethylene propylene, hexane, heptane, cycloalkanes, benzene, toluene etc.
Importance:
(i) Petrochemicals are very useful for providing raw materials for the manufacture of dyes, drugs, polymers, detergents, food preservatives, disinfectants etc.
(ii) These petrochemicals are widely used in the manufacture of iso-octane, plastics (polythene, PVC), synthetic rubber, insecticides, pesticides, explosives etc.
What are the necessary conditions for any system to be aromatic?
The compounds possessing aromatic character show the following characteristics:
(i) The compounds must be cyclic in nature and have flat planar structure.
(ii) Their molecular formulae suggest these compounds to be highly unsaturated due to the presence of one or more double bonds in the ring but they must behave as saturated compounds.
(iii) They must resist addition reaction and take part in the electrophilic substitution reactions.
(iv) The molecules have delocalised π electron cloud above and below the plane of the ring.
(v) The most essential criteria for the aromatic character is that the compound must obey Huckel’s rule. According to this rule, a cyclic compound will behave as aromatic compound if it contains (4 n + 2)  electrons, may be 0, 1, 2, 3 etc. Huckel's rule can be applied successfully to polycyclic compounds, annulenes and also other non-benzenoid compounds. For example;
electrons, may be 0, 1, 2, 3 etc. Huckel's rule can be applied successfully to polycyclic compounds, annulenes and also other non-benzenoid compounds. For example;
(a) Monocyclic systems: Some monocyclic systems having π-electrons (obey Huckel’s rule) possess aromatic character.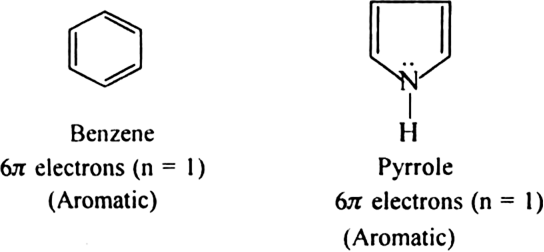
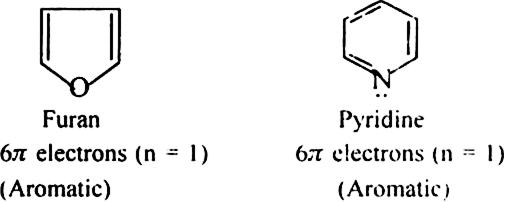
b) Fused ring systems: The polynuclear hydrocarbons such as naphthalene, anthracene and phenanthrene are also aromatic according to Huckel’s rule.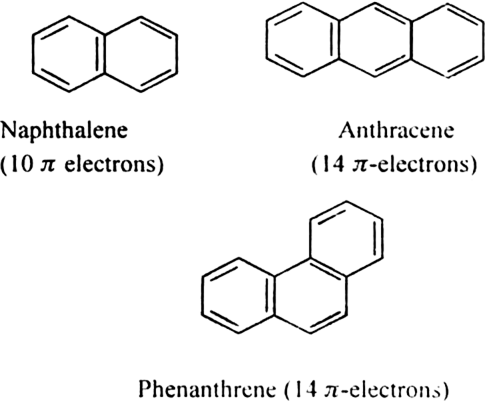
Aromatic ions: Some cyclic ions also exhibit aromatic character. For example.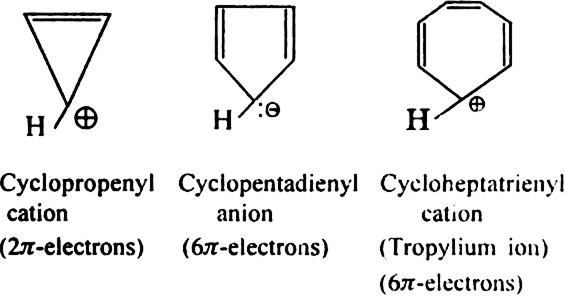
The following compounds are not aromatic: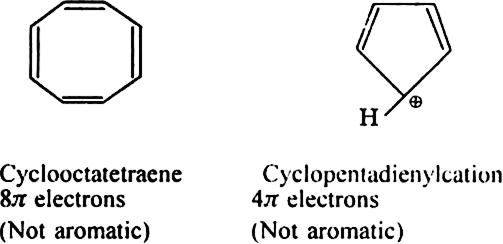
Cycloheptatriene although obeys Huckel’s rule yet it is not aromatic as it is not planar and can not show resonance.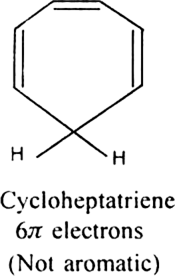
How will you convert benzene into m-nitrochlorobenzene .
How will you convert benzene into p-nitrotoluene.
Conversion of benzene into p-nitrotoluene.
Arrange benzene, n-hexane and ethyne in decreasing order of acidic behaviour. Also give a reason for this behaviour.
Hydrocarbon: Ethyne > Benzene > n-Hexane
Hybridization of C-atoms: (sp) (sp2) (sp3)
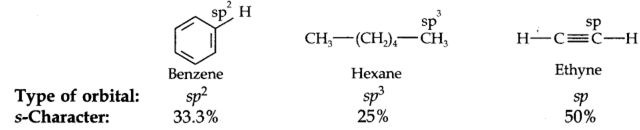
The acidic character is linked with the percentage of s-character. Greater the s-character, more is the electronegativity of the carbon atom and more will be the acidic character.
The correct statement regarding the comparison of staggered and eclipsed conformations of ethane is
-
The eclipsed conformation of ethane is more stable than staggered conformation because eclipsed conformation has no torsional strain
-
The eclipsed conformation of ethane is more stable than staggered conformation even though the eclipsed conformation has torsional strain
-
The staggered conformation of ethane is more stable than eclipsed conformation because staggered conformation has no torsional strain
-
The staggered conformation of ethane is less stable than eclipsed conformation, because staggered conformation has torsional strain.
C.
The staggered conformation of ethane is more stable than eclipsed conformation because staggered conformation has no torsional strain
Due to the absence of torsional strain staggered conformation of ethane is more stable than eclipsed conformation of it.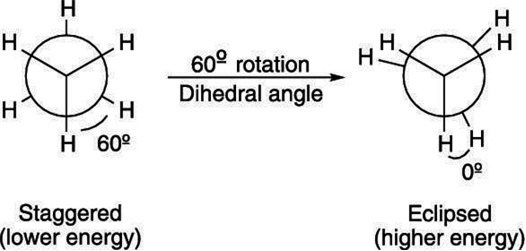
The IUPAC name of the compound  is
is
-
pent - 4 yn - 2- ene
-
pent - 3-en - 1-yne
-
pent - 2- en-4 -yne
-
pent - 1- yn-3- ene
B.
pent - 3-en - 1-yne
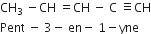
Mock Test Series
Sponsor Area
Sponsor Area






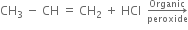
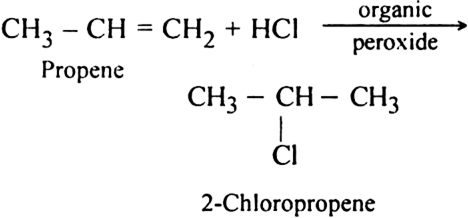
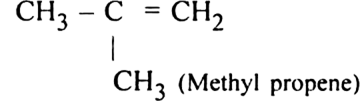
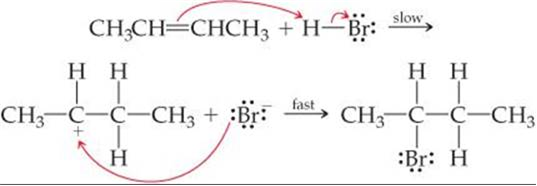
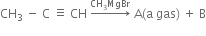
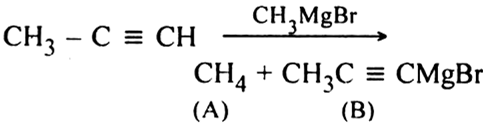
 bonds are present in the following structure
bonds are present in the following structure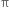 bonds.
bonds.