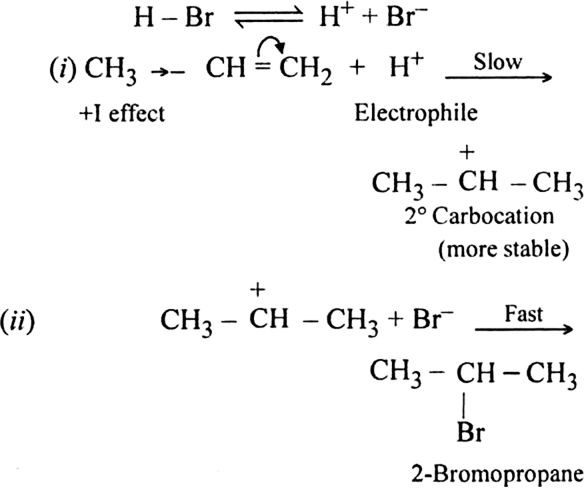
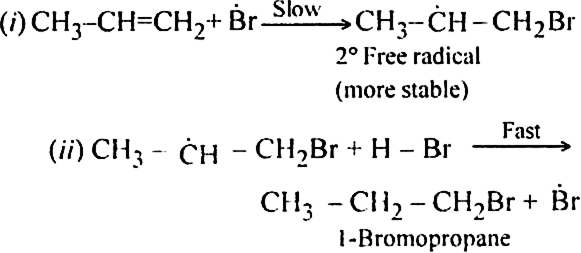The addition of HBr to propene yields 2-broniopropane, while in the presence of benzoyl peroxide, the same reaction yields 1-bromopropane. Explain and give mechanism.
Formation of 1-Bromopropane:
In the presence of benzoyl peroxide, the addition of HBr to propene involves free radical mechanism in which Br-free radical is obtained by the action of benzoyl peroxide on HBr.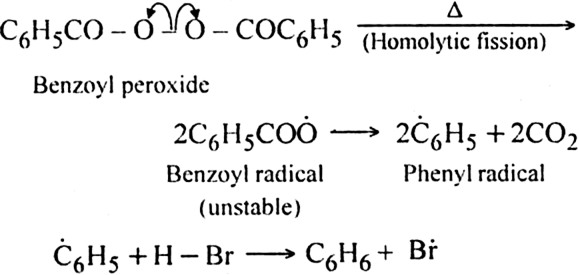
(i) Br radical adds to propene to form the more stable 2° free radical.
(ii) Free radical thus obtained rapidly abstracts a hydrogen atom from HBr to form 1-bromopropane.