Discuss the structure of benzene with special reference to Kekule structure.
The molecular formula of benzene is C6H6 indicating a high degree of unsaturation. As a result, benzene is expected to be highly reactive compound like alkenes or alkynes. Therefore benzene should:
(i) decolourise aqueous potassium permanganate.
(ii) add water in the presence of acids.
(iii) decolourise bromine water.
But actually, benzene does not undergo such reactions. This implies that benzene is different from alkenes and alkynes. Moreover, being extraordinarily stable, it behaves more like alkanes than an alkene or alkynes. It prefers to undergo substitution reactions rather than addition.
Since benzene on catalytic hydrogenation forms cyclohexane (a ring compound), this suggests that the carbon skeleton of benzene should be a six-membered cyclic structure.
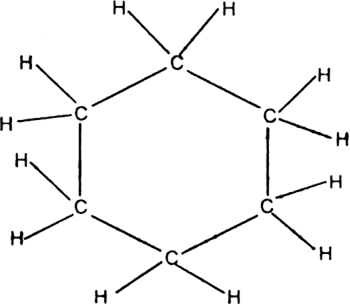
Kekule’s structure of benzene: Kekule proposed that benzene has a ring structure in which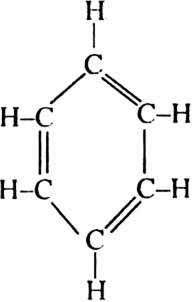
the six carbon atoms are arranged in the form of a regular hexagon and one hydrogen each is bonded to each carbon atom. Since carbon is tetravalent, he proposed that alternate single and double bonds are present between carbon atoms.
Objections to Kekule’s structure:
(i) Kekule’s structure allows the existence of two isomeric orthos-substituted dibromo benzene (I and NY).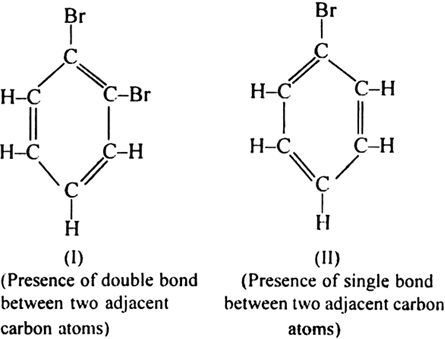
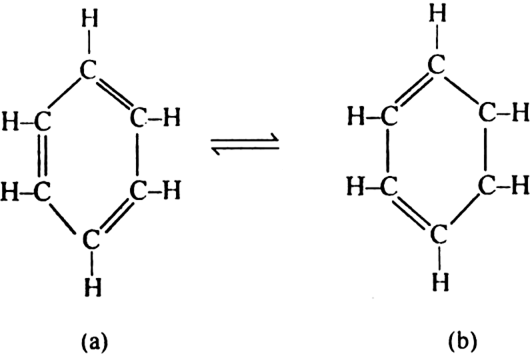
(ii) Kekule’s structure does not explain the extra ordinary stable nature of benzene molecule and its lack of reactivity towards addition reactions, resistance towards oxidation etc.
(iii) Equivalence of all the carbon-carbon bond lengths in benzene.



