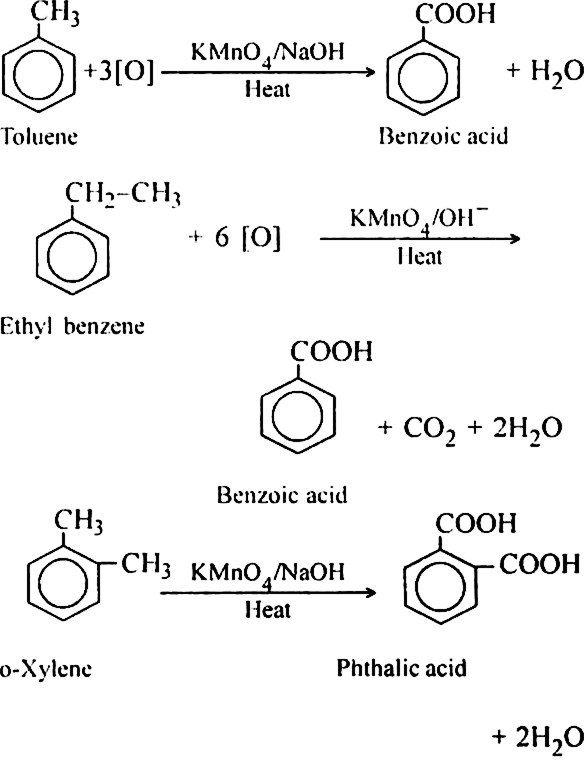
Discuss the oxidation reactions of arenes.
(i) Combustion: When burnt in oxygen, benzene and other hydrocarbons burn with a smoky flame and produce carbon dioxide and water.
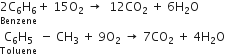
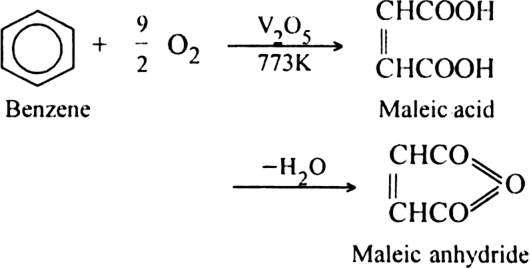
(ii) Oxidation of benzene: When a mixture of air and vapours of benzene is passed over vanadium pentoxide (V2O5) at 773 K, maleic anhydride is produced.
(iii) Oxidation of the side chain.
(a) With weak oxidising agent. Toluene is oxidised to benzaldehyde by using chromyl chloride (CrO2Cl2) or acidic manganese dioxide.
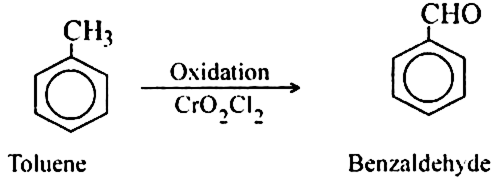
(b) With normal oxidising agent. On oxidation with a strong oxidising agent such as hot KMnO4, concentrated HNO3, acidified KMnO4 or acidified dichromate etc., the entire side chain, regardless of length, is oxidised to - COOH group.