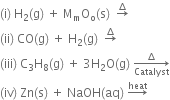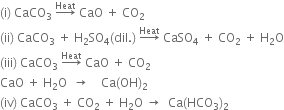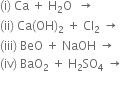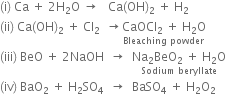Chemistry Part Ii Chapter 10 The S-Block Elements
Sponsor Area
NCERT Solution For Class 11 Business%252525252bstudies Chemistry Part Ii
What are s-block elements?
Why are alkali metals not found in nature?
Alkali metal has one electron each in the valence subshell of their atoms. Since they have only one electron in valence subshell, therefore, they lose easily, owing to their low ionisation energies. Therefore, alkali metals are highly reactive chemically and do not exist in the free or native state.
Lithium shows similarities to magnesium in the chemical behaviour. What is the cause of this similarity?
It is due to similarity in their:
(i) charge/size ratio.
(ii) ionic sizes.
Group 1 elements are called alkali metals. Why?
Why caesium can be used in photoelectric cell while lithium can not be?
In aqueous solution, Li+ ion has lowest mobility. Why?
Lithium has highest ionisation energy in group 1 elements, yet it is strongest reducing agent. Comment.
It is due to high hydration energy of small lithium ion which more than compensates high ionisation enthalpy. The hydration energy of Li+ ion is very high and as a result of this reduction potential of lithium is very low(E°= –3). So lithium acts as the most powerful reducing agent in aqueous solution.
The softness of group 1 elements increases down the group with increasing atomic number. Give reason.
Which is the most reactive alkali metal and why?
Caesium is the most reactive alkali metal, as it has lowest first ionisation enthalpy and lowest electron negativity.
Why alkali metals do not form M2+ ions?
It is because after the loss of one electron, alkali metal ion (M+ ) is formed which has stable noble gas configuration and it is very difficult to remove another electron to form M2+ ion as the second ionisation enthalpy is very high. For example, when lithium loses one electron and forms Li+ which is similar to Helium atom.
Name the radio active element in group 1. How does it resemble with the remaining elements of the group?
Why alkali metals are normally kept in kerosene oil?
This is because in the air they are easily oxidised to oxides which may dissolve in the moisture of the air to form hydroxides or they also combine directly with water vapours and catch fire.
Why lithium cannot be stored in kerosene?
Lithium cannot be stored in kerosene oil because it is the lightest metal and it floats on its surface and thus reacts with air.
Why does lithium form complexes?
Lithium form complexes because it is comparatively smaller in size and has a high tendency to accept electrons.
Why are alkali metal ions diamagnetic and colourless?
Why are alkali metals the most electropositive in nature?
Sponsor Area
Find out the oxidation state of sodium in Na2O2.
Oxidation state of Oxygen Na2O2 is =(-1)
Let x be the oxidation state of Na in Na2O2. Since peroxide linkage is present in Na2O2 in which the oxidation of O is –1.
Hence oxidation state of Na in  is +1.
is +1.
Explain why is sodium less reactive than potassium.
What happens when Magnesium is burnt in the air ?
When magnesium is burnt in air, dazzling brilliant light is given out and a mixture of magnesium oxide and magnesium nitride is formed.
Why do alkali metals form blue coloured solution with NH3?
Alkali metals form a blue coloured solution with ammonia due to the formation of ammoniated electron.
Why sodium and potassium are not found in the native state?
Tips: -
What happens when a piece of sodium is put in water? Give also balanced equation for the reaction.
Hydrogen gas is liberated.
Why sodium catches fire when dropped in water?
Sodium reacts with water with vigorous evolution of hydrogen which catches fire.
Name the alkali metals which form superoxides when heated in excess of air.
Which of the following alkali metal is having least melting point?
(i) Na (ii) K (iii) Rb (iv) Cs
Which one of the following alkali metal gives hydrated salts?
(i) Li (ii) Na (iii) K (iv) Cs
Arrange the following in decreasing order of ionic character: MF, MCl, MBr, MI where M reprents a metal.
Name the method for the extraction of sodium metal.
Sodium metal is extracted by Down's process
What is electrolyzed in Down’s process to obtain sodium?
Why does table salt get wet in rainy season?
Table salt is crude sodium chloride and contains impurities of calcium chloride, magnesium chloride and magnesium sulphate. Since these are deliquescent in nature they absorb moisture during the rainy season from the air. As a result, table salt gets wet.
What is the difference between soda ash and washing soda?
Give the formula of:
(i) baking soda (ii) salt cake?
(i) Baking soda: NaHCO3
(ii) Salt cake: Na2SO4.
How will you distinguish between sodium carbonate and sodium bicarbonate?
On heating, sodium bicarbonate decomposes to evolve CO2 while sodium carbonate does not.
Which alkali metal and which alkaline earth metal is radioactive? Give their atomic number also?
Fr(Z = 87); Ra(Z = 88)
Why does sodium hydride conduct current when melted?
Sponsor Area
Why does sodium hydride act as a reducing agent?

Why calcium chloride and potassium fluoride are added in the electrolysis of fused sodium chloride?
Why Be forms covalent compounds?
It is due to small atomic size and high value of ionisation enthalpy.
Why does a piece of burning magnesium ribbon continue to burn in sulphur dioxide?

Why do beryllium halides fume in moist air?
Beryllium halides on exposure to moist air undergo hydrolysis to form halogen acids. As a result, these halides fume in air.
Why alkaline earth metals do not occur in free state in nature?
Alkaline earth metals do not occur in the free state because of their high reactivity.
Which element of second group has maximum first ionisation enthalpy?
What is Mg+ ion unstable?
Mg+ ion unstable because Mg+ ion has still the tendency to lose another electron to form Mg2+ ion which has the stable noble gas configuration of the nearest inert gas (Neon).
What is oxone?
A mixture of Na2O2 and dilute HCl is called soda bleach, commercially known as oxone.
Write the correct sequence of alkaline earth metals in the group.
Sequence of alkaline earth metals in the group:
Be, Mg, Ca, Sr, Ba and Ra.
Potassium is more reactive than calcium. Explain.
Reactivity of an element depends on upon the ionisation enthalpy. Smaller the ionisation enthalpy, greater is the reactivity. As potassium has smaller ionisation enthalpy than calcium. Thus, potassium is more reactive than calcium.
Alkaline earth metals and their ions are smaller in size than those of group 1. Why?
It is due to increase in effective nuclear charge. Alkaline earth metals and their ions are smaller in size than those of group 1.
Alkaline earth metals have less electropositive character than the corresponding alkali metals. Why?
Alkaline earth metals have less electropositive character than the corresponding alkali metals because of higher ionisation enthalpy values of alkaline earth metals as compared to alkali metals.
Why solubility of alkaline earth metal compounds are comparatively less than the corresponding alkali metal compounds in water?
The solubility of alkaline earth metal compounds are comparatively less than the corresponding alkali metal due to the small size of bivalent ions of alkaline earth metals, the lattice enthalpy of alkaline earth metal compound is very high as compared to those of the metals of the first group.
Arrange the following in the increasing order of the property indicated:
KCl, MgCl2, CaCl2, BeCl2 - Ionic character.
BeCl2 < MgCl2 < CaCl2 < KCl.
Arrange the following in the increasing order of solubility in water: MgCl2, CaCl2, SrCl2, BaCl2.
Name the covalent compound from the following: NaCl, BeCl2, MgCl2.
BeCl2 is a covalent compound.
Compare the first and second ionisation enthalpies of sodium and magnesium.
First ionisation enthalpy IE1: Na < Mg
Second ionisation enthalpy : IE2: Na > > Mg
Which one is more basic Sr(OH)2 or Ba(OH)2?
Why does beryllium form BeCl2 although it has no unpaired electrons?
Be(Z = 4) which has electronic configuration 1s2 2s2 shows electronic configuration of 1s2 2s1 2px1 in excited state and one s-orbital (2s) and one p-orbital (2p) can intermix to form two sp hybrid orbitals which can be used to form BeCl2.
Which one of the alkaline earth metal carbonates is thermally the most stable?
- MgCO3
- CaCO3
- SrCO3
- BaCO3
D.
BaCO3 BaCO3. Since the electropositive character of the metal increases or the basicity of their hydroxides increases down the group, therefore their thermal stability increases. Hence BaCO3 is the most stable.What is diagonal relationship due to?
It is due to:
(i) similar size of atoms or ions
(ii) similar electronegativity and
(iii) similar polarising power.
Name the relationship which depicts the resemblance between Li and Mg.
Diagonal relationship
Which member of the alkaline earth metals family has maximum reduction potential?
Arrange the following in order of increasing basic character:
BeO, MgO, BaO, CaO.
BeO < MgO < CaO < BaO.
Potassium hydroxide is a stronger base than barium hydroxide. Explain.
Explain why an aqueous solution of beryllium chloride is acidic.
This is due to the hydrolysis of the beryllium ion.
Be2+ +2H2O --> Be(OH)2 + 2H+
What is 'Fluid Magnesia'?
A solution containing 12 g of MgCO3 per 100 cc of water containing dissolved CO2 is known as 'Fluid Magnesia'.
Halides of Be dissolve in organic solvents while those of Ba do not. Why is it so?
What is dead burnt plaster?
It is anyhydrous calcium sulphate obtained by heating gypsum above 393 K.
Why is anhydrous CaSO4 used as a drying agent? Why not Plaster of Paris?
Sponsor Area
Why anhydrous magnesium chloride is not obtained by simply heating hexahydrate?

What is magnesia cement or Sorel's cement?
The cement is a mixture of magnesium oxide (burnt magnesia) with magnesium chloride with the approximate chemical formula Mg4Cl2(OH)6(H2O)8, corresponding to a weight ratio of 2.5–3.5 parts MgO to one part MgCl2. A variant uses zinc oxide with zinc chloride instead of the magnesium compounds.
What happens when a burning magnesium ribbon is introduced into a jar of carbon dioxide?
It continues to burn.
Bones contain calcium ions. What do you think which anions are associated with them?
Bones are made of calcium phosphate.
Therefore, phosphate  ions are the ions associated with them.
ions are the ions associated with them.
What happens when calcium oxide is strongly heated with coke?
When calcium oxide(quick lime) is heated with coke in an electric furnance at 2273- 3273 K, calcium carbide (CaC2) is formed. 
Why is calcium preferred over sodium to remove last traces of moisture from alcohol?
Both Na and Ca react with water to form their respective hydroxides. In contrast, Na reacts with alcohol to form sodium ethoxide but calcium does not.
Therefore, calcium is preferred over sodium.
What is concrete?
It is a mixture of cement, sand, gravel (small pieces of stone) and appropriate amount of water.
What is fly ash?
It is a waste product from the steel industry and has properties similar to that of cement. It mainly consists of calcium silicate.
What are representative elements?
The elements of s-block and p-block are collectively called a representative or main group elements. The elements of groups 1 and 2 (s-block), 13 to 18 (p-block) constitute the main group elements or representative elements.
Name the elements of group 1. Write their electronic configurations.
The metallic elements lithium (Li), sodium (Na), potassium(K), rubidium (Rb), caesium (Cs) and francium (Fr) constitute group 1 of the periodic table. Francium is a radioactive element. They are known as alkali metals because their hydroxides are strong alkalies or bases.
Electronic configuration: The atoms of alkali metals have one electron in s-orbital outside a noble gas core. Therefore, their general electronic configuration is:
[Noble gas]ns1 where n = 2 to 7
The electronic configurations of alkali metals are given below:
| Element | At No | Electronic configuration |
| Lithium (Li) | 3 | [He]2s1 |
| Sodium (Na) | 11 | [Ne] 3s1 |
| Potassium (K) | 19 | [Ar] 4s1 |
| Rubidium (Rb) | 37 | [Kr] 5s1 |
| Caesium (Cs) | 55 | [Xe] 6s1 |
| Francium (Fr) | 87 |
[Rb] 7s1 |
Discuss the general trends in atomic and ionic radii of alkali metals.
The atoms of alkali metals have the largest size in their respective periods. The atomic radii increase on moving down the group among the alkali metals.
Reason: On moving down the group, there is a progressive addition of new energy shells. Although, the nuclear charge also increases down the group yet the effect of the addition of new shells is more predominant.
The radii of positive ion are smaller as compared to that of parent atom. Within the group, the ionic radii increase with the increase in atomic number.
The ionic radii of alkali metal ions in aqueous solution follows the order Li+ > Na+> K+ > Rb+ > Cs+ Justify the above order.
Discuss the general trends in ionisation enthalpy and electropositive character of alkali metals.
Alkali metals possess lowest ionisation enthalpies in their respective periods. However, within the group, the ionisation enthalpies of alkali metals decrease down the group.
Reason: The atoms of alkali metals are largest in their respective periods, therefore, the outermost electrons, which are far away from the nucleus, experience a less force of attraction with the nucleus and hence can be easily removed. Decrease in ionisation enthalpy, on moving down the group, is due to the increase in the size of the atoms of alkali metals and increase in the magnitude of screening effect is by virtue of an increase in the number of intervening electrons.
Electropositive character: On account of their low ionisation enthalpies, these metals have a strong tendency to lose their valence electrons and thus change into positive ions. Consequently, alkali metals are strongly electropositive or metallic in character. As this tendency for losing electron increases down the group, the electropositive character increases.
Why do alkali metals impart characteristic colours to the flame?
Why do alkali metals from unipositive ions?

Lithium ion has the lowest and caesium ion has the highest mobility in an electric field. Explain.
When an alkali metal dissolves in liquid ammonia the solution can acquire different colours. Explain the reactions for this type of colour change.
Or
Explain why alkali metals dissolve in liquid ammonia to form deep blue solution.

The blue colour of the solution is due to the ammoniated electrons.
Explain why alkali metals are electrically conducting and form soluble salts ?
Alkali metals have metallic bonds in which the positive ions are held together by electrons. The valence electrons move freely from one metal ion to another without much difficulty and thus have high electrical conductivity. Solubility depends on lattice enthalpy and hydration enthalpy. Since the alkali metal ions have low lattice enthalpy and high hydration energy so they form soluble salts.
How will you explain that alkali metals are chemically highly receive?
Due to low values of first ionisation enthalpies, low heats of atomization and high hydration enthalpies, alkali metals lose their valence electron readily and hence are chemically highly reactive.
Their relative tendency to lose electron in chemical reactions depends on upon:
(i) Ionisation enthalpy: Lower the ionisation enthalpy, greater the chemical reactivity.
(ii) Hydration enthalpy: Larger the hydration enthalpy, greater the chemical reactivity.
Now ionisation enthalpies of alkali metals decrease down the group and hence chemical reactivity increases. Also, hydration enthalpies of alkali metals increase down the group and so chemical reactivity increases. Hence, the chemical reactivity increases down the group of alkali metals. Thus, caesium (Cs) is the most reactive among the alkali metals i.e. Cs has maximum tendency to lose the valence electron to form monovalent Cs+ ions.
Why are potassium and caesium, rather than lithium used in photoelectric cells?
How will you explain that alkali metals are strong reducing agents?
Lower the ionisation enthalpy, greater is the tendency of an element to lose electrons and hence stronger is the reducing character or higher is the reactivity of the element. Since the ionisation enthalpies of alkali metals decrease down the group, therefore, their reducing character or reactivity in the gaseous state increases from Li to Cs i.e. Li < Na < K < Rb < Cs. However, in the aqueous solution, it has been absorbed that the reducing character of alkali metals follows the sequence Na < K < Rb < Cs < Li. In other words, lithium is the strongest reducing agent in aqueous solution.
Lithium is the strongest reducing agent in aqueous solution. Explain.
Electrode potential is the measure of the tendency of an element to lose electrons in the aqueous solution. Thus, more negative is the electrode potential, higher is the tendency of the element to lose electrons and hence stronger is the reducing agent.
Since the standard electrode potential  of
of
alkali metals become more and more negative as we move down the group from Na to Cs, therefore, reducing character of these elements increases in the same order i.e. Na to Cs. However, standard electrode potential (reduction) of lithium is the lowest i.e. -3.05 volts. In other words, lithium is the strongest reducing agent in the aqueous solution.
Reason. Electrode potential depends on :
(i) heat of sublimation
(ii) ionisation enthalpy
(iii) heat of hydration.

The sublimation enthalpies of alkali metals are almost similar. Now since Li+ ion is smallest in size, therefore, the large amount of energy released in step III (heat of hydration) compensates for the higher ionisation enthalpies, thereby facilitating the release of electron and hence explains the low value of electrode potential 
Why do alkali metals form ionic compounds?
This is because they have a strong tendency to lose the valence s-electron to acquire the nearest inert gas configuration. Lithium, however, because of its high ionisation enthalpy forms covalent compounds i.e. alkyl lithium (R - Li), aryl lithium (Ar - Li) etc.
why is melting point of lithium higher than sodium ?
It is because they have a strong tendency to lose the valence s-electron to acquire the nearest inert gas configuration. Lithium, however, because of it high ionisation enthalpy forms covalent compounds i.e. alkyl lithium (R – Li), aryl lithium (Ar – Li) etc.
Down the group new shell added which cause shielding effect and thus cause lower the melting point.
Why the melting point of sodium is lesser than that of lithium?
Why ionic conductance of alkali metal ions in aqueous solution are in the order:
Li+ < Mg + < K+ < Rb+ < Cs+ Explain ?
Why are lithium salts commonly hydrated and those of the other alkali metal ions usually anhydrous?
Lithium salts are commonly hydrated like LiCl.2H2O whereas other alkali ions are usually anhydrous. The hydration enthalpy of Li+ ion is maximum hydrated and therefore, the effective size of Li+ in aqueous solution is the largest. The hydration enthalpy decreases with increase in ionic size.
Li+ >Na+>K+> Rb+>Cs+
Therefore, the ions of other alkali metals are usually anhydrous.
Comment on each of the following observations:
The mobilities of the alkali metal ions in aqueous solution are Li+ < Na+ < K+ < Rb+ < Cs+
Li+ > Na+ > K+ > Rb+ > Cs+ therefore, ionic mobility increases in the order Li+ < Na+ < K+ < Rb+ < Cs+.
Comment on each of the following observations:
Lithium is the only alkali metal which forms nitride directly.
Lithium is the only alkali metal which forms a nitride directly Li+ and N3- ions are very small, so they form stable Li3N.

Lithium forms normal oxide, sodium forms peroxides while K, Rb and Cs form superoxides. Explain.
Lithium forms normal oxide  Lithium-ion with small size has a strong positive field around it. On combination with oxide anion, the positive field of lithium ion restricts the spread of negative charge towards another oxygen atom and thus prevents the formation of a higher oxide.
Lithium-ion with small size has a strong positive field around it. On combination with oxide anion, the positive field of lithium ion restricts the spread of negative charge towards another oxygen atom and thus prevents the formation of a higher oxide.
Sodium reacts with dioxygen to form sodium peroxide  Sodium ion with a larger size than lithium ion has weaker positive field than lithium ion. This positive field is so weak that it cannot prevent the conversion of the oxide anion
Sodium ion with a larger size than lithium ion has weaker positive field than lithium ion. This positive field is so weak that it cannot prevent the conversion of the oxide anion  into a peroxide ion
into a peroxide ion  , However, it is strong enough to prevent further oxidation of peroxide to superoxide.
, However, it is strong enough to prevent further oxidation of peroxide to superoxide.
Potassium, rubidium and caesium react with dioxygen to form superoxide 
Potassium, rubidium and caesium ions are large sized and thus have a very weak positive field around them. The positive field around these ions is so weak that it cannot prevent the conversion of peroxide  anion to superoxide anion
anion to superoxide anion 
'Strenth of the bases increases from LiOH to CsOH'. Comment
Write two properties of lithium carbonate in which it differs from other alkali metal carbonates.

(ii) Lithium carbonate is less soluble in water as compared to carbonates of other alkali metals.
Why is Li2CO3 decomposed at a lower temperature while Na2CO3 at higher temperature?

On the other hand, Na2CO3 is a salt of a strong base (NaOH) and a weak acid (H2CO3). Since NaOH is much stronger base than LiOH and hence can attract CO2 more strongly. Therefore Na2CO3 decomposes at much higher temperature because it is more stable than Li2CO3.
Why is LiF almost insoluble in water whereas LiCl in soluble not only in water but also in acetone?
LiF is almost insoluble in water because of much higher lattice energy than that of LiCl. Since Li+ ion (small size) can polarise bigger Cl- ion more easily than the smaller F- ion, therefore, according to Fajan rules, LiCl has more covalent character than LiF and hence LiCl is soluble in the organic solvents like acetone.
How would you explain that the following observations?
LiI is more soluble than KI in ethanol?
As the size of Li+ ion is much smaller than K+ ion, therefore Li+ ion can polarise bigger I- ions to a greater extent than K+ ions (Fajan rule). As a result, LiI is more covalent than KI and hence is more soluble in ethanol. (organic solvent).
Explain: Lithium exhibits anamalous behaviour in the company of alkali metals.
Or
Name the chief factor responsible for the anomalous behaviour of lithium.
Or
List three properties of lithium in which it differs from the rest of the alkali metals.
Anomalous behaviour of lithium is due to its:
(i) very small size,
(ii) high electronegativity and ionisation energy enthalpy value and
(iii) the absence of d-orbitals in the valence shell of its atom.
Therefore, lithium differs from other members of the family in the following respects:
(i) Lithium is harder than other alkali metals.
(ii) Lithium combines with oxygen to form lithium oxide while other alkali metals form peroxides and superoxides.
(iii) Lithium when heated with ammonia forms imide, Li2NH, while other alkali metals form amides, MNH2 as:
(iv) Lithium hydroxide and lithium carbonate decompose on heating while the hydroxides and carbonates of other alkali metals do not decompose on heating.
(v) Lithium unlike other alkali metals from no ethynide on reaction with ethyne.
(vi) Lithium nitrate when heated gives lithium oxide, while other alkali metal nitrates decompose to give the corresponding nitrites. 
How will you explain the reactivity of alkali metals with halogens ?
Alkali metals combine with halogens to form metal halides, which are ionic crystalline solids having general formula M+X–.
Reactivity of alkali metals with particular halogen increases from Li to Cs. On the other hand, the reactivity of halogens with particular alkali metal M decreases from F2 to I2.
How will you explain the ionic character of alkali metal halides?
Ionic character of alkali metal halides: When a cation approaches an anion, the electron cloud of the anion is attracted towards the cation, thus it gets distorted or polarised. The capacity of the cation to polarise the anion is called polarising power, and the tendency of the anion to become polarised, is known as its polarizability. Now greater the polarisation caused, greater is the neutralisation of charge and consequently the ionic character is decreased (or covalent character is increased). The polarising power of a cation and the polarizability of an anion are determined in term of following.
Fajan’s rules:
(i) Cation’s size: Smaller is the cation, greater is its polarising power, e.g. LiCl is less ionic (or more covalent) than KCl, because the size of Li+ ion is much smaller than that of K+ ion.
(ii) Anion’s size: Larger is the anion, higher is its polarizability, since the hold on the electron-cloud by the nucleus of anion decreases. Hence, the ionic character of lithium halides is in the order : LiF > LiCl > LiBr > Lil.
Alternatively, the covalent character is in the order Lil > LiBr > LiCl > LiF. Since higher the ionic character, higher is the melting point, consequently melting point of LiF > LiCl > LiBr > Lil.
In what ways lithium shows similarities to magnesium in its chemical behaviour?
Or
List four properties to show the diagonal relationship between lithium and magnesium.
The similarity between Li and Mg is because of their similar atomic radii (Li = 152 pm; Mg = 160 pm) and ionic radii. (Li+ = 76 pm, Mg2+ = 72 pm).
These two elements resemble each other in the following properties:
(i) Both Li and Mg decompose water very slowly with the liberation of hydrogen.
(ii) Both Li and Mg form nitrides - Li directly and Mg on burning in nitrogen.
(iii) LiOH and Mg(OH)2 are weak bases.
(iv) Nitrates of both decompose on heating to give oxides.

(v) Both LiCl and MgCl2 are soluble in ethanol.
(vi) Both LiCl and MgCl2 are deliquescent and crystalline from aqueous solution as hydrates, LiCl2. 2H2O and MgCl2.8H2O.
Sponsor Area
How does lithium occur in nature? Name the chief ores of lithium.
Lithium mainly occurs as silicate minerals but the amount present in any mineral is always small and thus extraction of the metal is not so easy. The chief ores of lithium are:
(i) Spodumene: LiAl(SiO3)2 containing 4·6% lithium.
(ii) Triphylite (Li, Na)3PO4 (Fe, Mn)3(PO4)2 containing up to 4% lithium.
(iii) Petalite, LiAl (Si2O5) containing 2·7 3·7% lithium.
(iv) Lepidolite, (Li, K, Na)2Al2(SiO3)2 containing 1·5% lithium.
Traces of lithium are also present in milk, blood, plants etc.
Discuss the extraction of lithium.
It involves the following steps:
(i) Preparation of lithium chloride: The silicate mineral is crushed to fine powder and then boiled with dilute H2SO4 and filtered to remove insoluble silica (SiO2). The mother liquor is then treated with calculated amount of Na2CO3 to precipitate aluminium and iron as carbonates which are filtered off. The filtrate is then treated with an excess of Na2CO3 to precipitate lithium as Li2CO3. The precipitate is dissolved in HCl to form LiCl.
(ii) Electrolysis of lithium chloride. A mixture of dry lithium chloride and potassium chloride is fused and electrolyzed in an electrolytic cell (Down’s cell). Potassium chloride is added to increase the electrical conductivity and also to lower the melting point from 883K to 723K. The cell is operated at a temperature of about 675-775K and voltage of 8-9 volts is applied.
As a result of electrolysis, the following reactions take place:
Chlorine gas is liberated at the anode while molten lithium rises to the surface of the fused electrolyte and collects in the cast iron enclosure surrounding the cathode. The metal thus obtained is 99% pure.
What difficulties arise in the extraction of lithium?
The lithium metals can not be extracted by the usual procedure due to the following reasons:
(i) The metal can not be obtained by the reduction of its oxide (Li2O or Na2O) because it is a strong reducing agent by itself and the common reducing agents such as carbon, hydrogen, magnesium and aluminium cannot be used.
(ii) Metal can not be obtained by the electrolysis of the aqueous solution of its salt like LiCl because the metal formed at the cathode will violently react with water to form lithium hydroxide and hydrogen.
(iii) Even molten LiCl can not be used for the electrolysis because the melting point of the salt is so high that it is quite difficult to attain and maintain this temperature.
(iv) Chlorine, a by-product of electrolysis, will corrode the material of the vessel at this higher temperature.
What happens when:
(i) Lithium reacts with air
(ii) Lithium reacts with water
(iii) Lithium reacts with halogen
(iv) Lithium reacts with acids?

(ii) Lithium reacts with water slowly to evolve hydrogen.

(iii) Lithium form halides on reacting with halogen.
 .
.The halides of lithium are mostly covalent in nature.
(iv) Lithium reacts with dilute HCl and dilute H2SO4 to evolve hydrogen gas.

The reaction with concentrated H2SO4 is highly exothermic and the metal catches fire.
Account for the following:
(i) Lithium can not form monovalent cation (Li+) easily.
(ii) Lithium iodide is more covalent than lithium fluoride.
(i) The ionisation enthalpy of lithium is maximum in the group. Therefore, it can not form monovalent cation (Li+) so easily as compared to the other alkali metals.
(ii) According to Fajan rule, Li+ ion can polarise I– ion more than the ion because of the bigger size of the anion. Therefore, lithium iodide has more covalent character than lithium fluoride.
Name a few important uses of lithium.
Important uses of lithium:
(i) Lithium is used as deoxidiser in the purification of copper and nickel.
(ii) Lithium is used as a getter or scavenger.
(iii) Lithium bromide is used in medicines as a sedative.
(iv) Lithium carbonate is used in making a special variety of glass.
(v) Lithium aluminium hydride (LiAlH4) is used as a reducing agent in synthetic organic chemistry.
How does sodium occur in nature? Name the chief ores of sodium.
Occurrence: Sodium does not occur in nature in the free state due to its very reactive nature. In the combined state, it occurs to the extent of about 2.6% in the earth's crust. The main ores of sodium are:
1. Sodium chloride (NaCl) found as rock salt and in sea water.
2. Sodium nitrite (NaNO3) or Chile saltpetre.
3. Sodium carbonate (Na2CO3)
4. Borax (Na2B4O7 . 10H2O).
What difficulities arise in the extraction of sodium? How these difficulties are overcome?
Difficulties in the extraction of sodium: The sodium metal cannot be extracted by the usual procedure due to the following reasons:
1. Sodium is a very strong reducing agent and it cannot be obtained by the reduction of sodium oxide with a reducing agent such as carbon.
2. It cannot be prepared from its aqueous solution by metal displacement method as the liberated metal reacts with water.
3. The metal cannot be isolated by the electrolysis of the aqueous solution of its salt such as NaCl because the sodium formed at cathode at once reacts with water producing sodium hydroxide and liberates hydrogen gas.
The above difficulties are solved by carrying out the electrolysis of sodium chloride containing some calcium chloride and potassium fluoride also in the molten state.
Molten sodium chloride cannot be used due to the following reasons:
(i) The melting point of sodium chloride (1085K) is very high and it is quite difficult to keep the sodium chloride in the molten state at such a high temperature during its electrolysis.
(ii) Since the boiling point of sodium is 1160K, the metal tends to change into vapours at a high temperature of electrolysis.
(iii) At the high temperature of electrolysis, both liberated sodium and molten sodium chloride tend to form a fog which is very difficult to separate.
(iv) The product of electrolysis (sodium and chlorine) can react chemically with the apparatus used for electrolysis at high temperature.
The main function of calcium chloride and potassium fluoride is to lower the melting point of sodium chloride from 1085K to 873K. This is because the above-mentioned difficulties are only due to the high melting point of sodium chloride.
Discuss Down's process for the isolation of sodium.
In this method, sodium is prepared by the electrolysis of fused anhydrous sodium chloride. Some calcium chloride and potassium fluoride are added to it. It lowers the melting point of sodium chloride from 1085 to 873K. The electrolysis is carried out in an iron box lined with fire bricks known as Down’s cell.
It consists of a graphite anode projected up through the bottom of the cell which is surrounded by a cylindrical iron cathode. The anode and cathode are separated by a wire gauze shell through which molten sodium chloride can easily pass but melted sodium cannot. The cell is also produced with a storage drum for receiving molten metal, an outlet for the removal of chlorine gas at the top.
On electrolysis, chlorine is liberated at the anode which escapes through the iron hood at the top. Sodium is liberated at the cathode and remains in the wire gauze shell. The level of molten sodium rises and it overflows into the receiver. The following reactions take place:
Sodium metal obtained by this method is about 99.5% pure.
Why is sodium kept under kerosene oil?
Sodium cannot be kept in the air because its surface gets tarnished due to the formation of a layer of its oxide, hydroxide and carbonate on its surface.

Sodium cannot be kept in water because it reacts with water violently and hydrogen evolved catches fire.

Therefore, sodium is kept in kerosene oil.
What happens when:
(i) sodium reacts with hydrogen halide,
(ii) sodium reacts with acetylene,
(iii) sodium is heated with hydrogen and
(iv) sodium is treated with mercury ?
(i) When sodium is treated with hydrogen halide, hydrogen gas is evolved.

(ii) Sodium reacts with acetylene to form sodium acetylide.

(iii) When sodium is heated with hydrogen, sodium hydride is produced.

(iv) When sodium is treated with mercury, sodium amalgam is formed as the product.

What happens when:
(i) sodium metal is dropped in water?
(ii) sodium metal is heated in free supply of air?
(iii) sodium peroxide dissolves in water?

(ii) When sodium metal is heated in a free supply of air, sodium peroxide (Na2O2) along with small amount of sodium oxide is formed.

(iii) When sodium peroxide is dissolved in water, hydrogen peroxide is produced.

Account for the following:
(i) Sodium imparts colour to the flame.
(ii) Sodium acts as a strong reducing agent.
(i) Due to low ionisation energy, when sodium metal or its salt is heated in a flame, the valence electron is excited to higher energy level. When this excited electron returns to the ground state, the absorbed energy is emitted in the visible region of the electromagnetic spectrum and hence the flame appears coloured.
(ii) The reducing character of sodium is due to its low ionisation energy as a result of which it can easily lose its valence electrons and act as strong reducing agent. For example,

Name a few important uses of sodium ?
It is used:
(i) in sodium vapour lamps.
(ii) as a coolant in the valves of internal combustion engines and in nuclear reactions.
(iii) in drying organic solvents such as benzene to remove even last traces of moisture.
(iv) as a reagent for the detection of nitrogen, sulphur and halogens in the organic compounds.
(v) in sodium amalgam which is used as a reducing agent in organic synthesis.
(vi) in the preparation of sodium compounds such as Na2O2, NaCN, NaNH2.
(vii) in the manufacture of a sodium-lead alloy which is used in the manufacture of tetraethyl lead which is added to petrol as an antiknock compound.
Discuss in brief the Solvay process for the manufacture of washing soda.
Principle:The process is based upon the low solubility of sodium bicarbonate especially in the presence of CO2, ammonia and carbon dioxide are passed through a saturated solution of sodium chloride, sodium bicarbonate is produced.


Sodium bicarbonate formed is least soluble in the presence of an excess of carbon dioxide and NaCl (common ion effect) and hence filtered.
Sodium bicarbonate is then heated strongly to produce sodium carbonate, carbon dioxide (which is used again) and steam.

Ammonium is recovered by heating ammonium chloride solution with slaked lime.

Ammonia gas is used again.
(i) Common salt (NaCl)
(ii) Lime - stone (CaCO3)
(iii) Ammonia gas (NH3)
(iv) Coke for heating.
Process. The actual manufacture of sodium carbonate is carried out as follows:
1. Saturating or ammoniation tank. It is an upright iron cylinder having a conical base. The brine solution is taken in this tower and a mixture of ammonia gas and carbon dioxide is bubbled through it. Any impurities of calcium and magnesium salts in the brine solution are precipitated as carbonates and removed with the help of filter press.
2. Carbonating tower. It is provided with perforated horizontal plates. The clear ammoniacal brine after cooling flows downward slowly and carbon dioxide (from lime kiln) introduced at the base of the tower rises in small bubbles. Ammonical brine and carbon dioxide move in opposite directions and therefore, the two react on the principle of counter currents. Sodium bicarbonate is formed which is least soluble in excess of carbon dioxide and sodium chloride and hence precipitated.

3. Rotary vacuum filter. The milky liquid containing small crystals of sodium bicarbonate is drawn off at the base of the carbonating tower. It is filtered by means of a rotary vacuum filter and then scrapped off. The remaining liquor containing NH4Cl is pumped to the top of the ammonia recovery tower.
4. Calcination of sodium bicarbonate. The sodium bicarbonate is calcined in a covered pan or a rotary furnace. It undergoes decomposition to form sodium carbonate, carbon dioxide and stream.
Carbon dioxide set free is again used in the carbonating tower together with the gas(from lime kiln).
5. Limekiln. Here lime -stone is heated to get carbon dioxide and calcium oxide.

Carbon dioxide formed is passed through the base of carbonating tower to form NaHCO3. The CaO is treated with a large quantity of water to get Ca(OH)2 which is pumped on to the ammonia recovery tower.
6. Ammonia recovery tower. The filtrate from the vacuum filter (Step 3) containing ammonium chloride is heated (using steam) along with Ca(OH)2 (obtained from lime kiln) to get a mixture of ammonia with a small amount of carbon dioxide gas. 
The ammonia with a small amount of carbon dioxide evolved is returned to the saturating tank for re-use. CaCl2 is the only waste product of this process.
Thus, the ammonia-soda process is very cheap, self-contained and self-sufficient and sodium carbonate formed is quite pure.
Discuss the various reactions which occur in the Solvay ammonia process.
In this process, brine (i.e. a concentrated solution of NaCl), ammonia and carbondioxide are the raw materials. The chemical reactions involved are:
CO2 needed for the reaction is obtained by heating calcium carbonate and quick lime (CaO) is dissolved in water to form slaked lime Ca(OH)2.
NH3 needed for the reaction is obtained by heating NH4Cl formed in eq. (1) with Ca(OH)2 formed in eq. (2).
The only by product of the reaction is calcium chloride (CaCl2).
List some important uses of washing soda (sodium carbonate).
Important uses of washing soda:
1. It is used in the manufacture of glass, caustic soda, borax and soap powders.
2. It is used for softening water.
3. It is used in industries like textile, paper, petroleum, refining, paints etc.
4. It is used in laundries as washing soda.
5. It is used as a laboratory reagent.
Potassium carbonate cannot be prepared by Solvay process. Why?
This is due to the reason that potassium bicarbonate (KHCO3) formed as in intermediate (when CO2 gas is passed through ammoniated solution of potassium chloride) is highly soluble in water and cannot be separated by filtration.
Can we prepare potassium bicarbonate by Solvay process?
What is the action of heat on Na2CO3.10H2O?
On heating below 373K, it loses 9 molecules of water of crystallisation to form monohydrate (Na2CO3.H2O). On heating above 373K, the monohydrate changes to an anhydrous white powder called soda ash but does not decompose further.
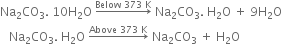
State as to why a solution of Na2CO3 is alkaline.

What happens when:
(i) sodium carbonate reacts with the milk of lime.
(ii) sodium carbonate is added to water.
(iii) sodium carbonate reacts with a dilute mineral acid?
 .
.(ii) It undergoes hydrolysis to form an alkaline solution.
 .
.(iii) It reacts with dilute mineral acid evolving CO2 gas.
 .
.How is sodium hydroxide manufactured? Discuss in brief the details of the process.
Or
With the help of a diagram, show the reactions at the cathode and anode in the manufacture of sodium hydroxide by the Castner - Kellner process.


Castner - Kellner cell consists of a rectangular iron tank. It is divided into three compartments by two non-porous state partitions. The two partitions suspended from the top almost reach the bottom of the cell, without touching it. A layer of mercury is placed at the bottom of the cell. The mercury in one compartment can flow into another compartment but the solutions in the compartment cannot intermix with one another. The cell is provided with an eccentric wheel at its bottom. The bottom layer of mercury can be put in motion with the help of this wheel.
The mercury at the bottom of the cell acts as an intermediate electrode by induction. It serves as the anode in the middle compartment and as a cathode in the outer compartments. These outer compartments are provided with graphite anodes. The saturated brine solution is put in them. The middle compartment contains dilute caustic soda. A series of iron rods fitted in this compartment act as a cathode. On passing electric current, the following reactions occur:
1. In the outer compartments. Sodium chloride solution is electrolyzed. Chlorine is liberated at the anodes. Sodium ions are discharged at the mercury cathode and metallic sodium forms. This combines with mercury forming sodium amalgam (Na-Hg). The sodium amalgam formed is transferred to the central compartment by giving a slight rocking motion to the cell.

At anode:

At cathode:
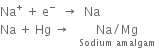
2. In the central compartment. Here sodium-amalgam acts as an anode, while the iron rods act as a cathode. The sodium amalgam reacts with water to form sodium hydroxide. Here, following reactions take place:
At Na-Hg anode.

At iron cathode.

Hydrogen escapes out through an outlet at the top. The strength of sodium hydroxide in the central compartment gradually increases, when it reaches a concentration of about 20%, it is removed and evaporated to get solid sodium hydroxide.
Name a few important uses of sodium hydroxide.
Sodium hydroxide is used:
(i) in the manufacture of metallic sodium, artificial silk and dyes.
(ii) in the refining of petroleum and as a reagent in the laboratory.
(iii) in soap, textile and paper industries.
(iv) in the preparation of soda lime (NaOH + CaO).
(v) for preparing bleaching agents like sodium hypochlorite and for the purification of bauxite.
Explain what happens when:
(i) Sodium hydrogen carbonate is heated
(ii) Sodium amalgam reacts with water
(iii) Fused sodium metal reacts with ammonia?
(i) When sodium hydrogen carbonate is heated, sodium carbonate is formed.

(ii) Sodium amalgam reacts with water liberating hydrogen gas.
(iii) When ammonium is passed through molten sodium, it yields sodamide evolving H2 gas.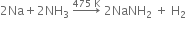
Explain the significance of sodium, potassium, calcium and magnesium in biological fluids ?
The significance of sodium and potassium in biological fluids: K+ and Na+ cations are present in the red blood cells. The ratio of K+ to Na+ ions in a mammal such as human beings, rabbit, rats and horses is 7:1. These cations accumulate in cells and create a concentration gradient and potential in the membrane. Electric pulse in the every is generated when a chemical is released during activation of discharge the membrane potential.
Magnesium is part of chlorophyll the green colouring matter of plants. Photosynthesis takes place only in the presence of chlorophyll.
Calcium is present in the form of calcium phosphate Ca3(PO4)2 in bones, Ca and Mg play a key role in the formation of phosphorus- oxygen linkage in biological systems for storage of energy. The pyrophosphate hydrolysis which releases energy is controlled by calcium ions. These ions perform important biological functions such as maintenance of ion balance and nerve impulse conduction.
State as to why sodium is found to be more useful than potassium.
Sodium ions which are present in blood plasma and in the interstitial fluid help:
(i) in the transmission of nerve signals.
(ii) in regulating the flow of water across cell membrane.
(iii) in the transport of sugars and amino acids. Thus sodium is found to be more useful than potassium (K+ ions are present in the cell fluids).
Industrial uses of sodium metal reflect its strong reducing power, about 60% of world production of sodium are used to male tetraethyl lead PbEt4 for the gasoline antiknocks.
i) It is also used in dye industry.
ii) It is also used for detecting the presence of nitrogen, Sulphur and halogen in organic compounds. It is widely used as sodium amalgam as a reductant.
Name the elements of group 2. Write down their electronic configurations ?
The metallic elements beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba) and radium (Ra) constitute group 2 of the periodic table. Radium is a radioactive element. These are known as alkaline earth metals.
Electronic configuration. The atoms of alkaline earth metals have two electrons in s-orbital outside a noble gas core. Therefore, their general electronic configuration is [Noble gas] ns2 where n = 2 to 7.
The electronic configuration of alkaline earth metals are given below:
| Element | At. No. | Electronic configuration |
| Beryllium (Be) | 4 | [He] s2 |
| Magnesium (Mg) | 12 | [Ne] 3s2 |
| Calcium (Ca) | 20 | [Ar] 4s2 |
| Strontium (Sr) | 38 | [Kr] 5s2 |
| Barium (Ba) | 56 | [Xe] 6s2 |
| Radium(Ra) | 88 | [Rn] 7s2 |
Why elements of group 2 are called alkaline earth metals?
The oxides of these elements of Group 2 were known much earlier than the metals themselves. These oxides were alkaline in nature and were found in the earth's surface. Thus, the elements of group 2 were given the name alkaline earth metals.
The alkaline earth metals are denser and harder than the corresponding alkali metals. Explain ?
The atoms of alkaline earth elements have smaller size and stronger intermetallic bonds, as compared to alkali metals. Therefore, they are more closely packed in their crystal lattices which account for high density and hardness of these elements.
In general, the density first decreases up to calcium and then increases up to radium. The case for a decrease in density up to calcium is presumably due to less good packing arrangement of constituents in the solid lattice.
What is the trend of atomic and ionic radii of alkaline earth metals within the group?
Within the group, the atomic as well as ionic radii increase in going from beryllium (Be) to radium (Ra). This is due to the addition of new shells, the effect of which is more predominate than the increase in nuclear charge.
The atomic and ionic radii of alkaline earth metals are smaller than those of the corresponding alkali metals. Explain.
The atoms of alkaline earth metals have a higher nuclear charge due to which the electron cloud is pulled towards the nucleus. This causes a decrease in the atomic and ionic radii of alkaline earth metals.
How will you explain that alkaline earth metals have much higher melting and boiling points than those of alkali metals?
Discuss the general trends in ionisation enthalpy of the alkaline earth metals.
i) The alkaline earth metals owing to their large size of atoms have fairly low values of ionisation enthalpies. Within the group, the ionisation enthalpy decreases as the atomic number increases. It is because of increase in atomic size due to the addition of new shells and increase in the magnitude of screening effect of the electrons in inner shells.
ii) The first ionisation enthalpies of alkaline earth metals are higher than alkali metals because of smaller atomic size and higher than alkali metals because of smaller atomic size and higher effective nuclear charge. Second ionisation enthalpy is smaller than that of alkali metals because alkali metals acquire noble gas configuration after losing one electron.
The first ionisation enthalpies of alkaline earth metals are higher than those of corresponding alkali metals. Explain.
Second ionisation enthalpies of alkali metals are much higher than those of the alkaline earth metals. Explain.
Alkali metals have only one valence electron, while alkaline earth metals have two valence electrons. After the removal of one electron from alkali metals, they form M+which is a stable noble gas configuration. Removal of another electron from the unipositive ion of alkali metal, therefore, would require a very large amount of enthalpy.
On the other hand, after the removal of one electron from alkaline earth metals, they do not acquire stable noble gas configuration and still have a tendency to lose another electron. As a result, the second ionisation enthalpies of alkali metals are much higher than those of the alkaline earth metal.
How will you explain the electropositive or metallic character of alkaline earth metals?
Alkaline earth metals are fairly electropositive as their atoms have a tendency to lose their two outermost electrons forming dipositive ions. 
On moving down the group, the atomic radii increase and ionisation enthalpies decrease. Consequently, the electropositive or metallic character increases. Thus, Mg is more electropositive than Be and so on.
Alkaline earth metals are less electropositive than the alkali metals. Explain.
Alkaline earth metals are less electropositive because of the smaller size and higher ionisation enthalpies of alkaline earth metals as compared to alkali metals.
Beryllium and magnesium do not give colour to flame whereas other alkaline earth metals do so. Why?
How will you explain the reducing character of alkaline earth metals?
Except beryllium, the alkaline earth metals have a fairly strong tendency to lose two electrons to form dipositive ions because of their low ionisation enthalpies and high negative value of standard electrode potentials. Therefore, they act as reducing agents.
The reducing character of alkaline earth metals increases as we move down the group from Be to Ba because the ionisation enthalpies increase and electrode potentials become more and more negative with increasing atomic number from Be to Ba.
Comment on each of the following observations:
(where M = Ca, Sr or Ba) is nearly constant.
 (i) Ionisation enthalpy
(i) Ionisation enthalpy(ii) Enthalpy of hydration
(iii) Enthalpy of vaporisation
The combined effect of these factors is approximately the same for Ca, Sr, and Ba. Hence, their electrode potentials are nearly constant.
Explain why alkaline earth metals are poor reducing agents as compared to alkali metals.
The ionisation enthalpies of alkaline earth metals are higher and their electrode potentials are less negative than the corresponding alkali metals, therefore alkaline earth metals are weaker reducing agents than alkali metals.
Explain the trend of solubility of carbonate, sulphates and hydroxides of alkaline earth metals ?
The solubility of carbonates and sulphates of these metals decreases downward in the group. This is because the magnitude of the lattice enthalpy remains almost constant as the carbonate or sulphate is so big that small increase in the size of the cations from Be to Ba does not make any difference. However, the hydration enthalpy decreases from Be2+ to Ba2+ sufficiently with the increase in their size resulting in the decrease of solubility of their carbonates or sulphates.
The solubility of hydroxides of these metals in water increases downward in the group. This is due to the fact that the lattice enthalpy decreases down the group due to increase in the size of the cation of the alkaline earth metal. On the other hand, the hydration enthalpy of the cations of alkaline earth metals decreases as we go down the group. As a result ∆Hsolution (∆Hlattice – ∆Hhydration) becomes more negative and solubility increases.
Account for the following:
(i) Be(OH)2 is amphoteric while Mg(OH)2 is basic.
(ii) Be(OH)2 is insoluble but Ba(OH)2 is fairly soluble in water.
(i) This is because I.E. of Mg < I.E. of Be. So bond M – OH can break more easily in Mg(OH)2 than in Be(OH)2.
(ii) This is because with the increase in size (from Be to Ba), the lattice enthalpy decreases significantly but hydration enthalpy remains almost constant.
The hydroxides and carbonates of sodium and potassium are easily soluble in water while the corresponding salts of magnesium and calcium are sparingly soluble in water. Explain ?
How would you explain that BeO is insoluble but BeSO4 is soluble in water?
BeO is covalent in nature due to its smaller size, high ionisation enthalpy and high electronegativity and therefore it is insoluble in water. On the order hand, BeSO4 is ionic. Also because of small size of Be2+ ion, the hydration enthalpy of BeSO4 is much higher than its lattice enthalpy. Thus BeSO4 is highly soluble in water.
How would you explain that BaO is insoluble but BeSO4 is soluble in water?
How would you explain that BaO is insoluble but BeSO4 is soluble in water?
Explain why halides of beryllium fume in moist air but other alkaline earth metal halides do not.

What is meant by the diagonal relationship of elements? Discuss the diagonal relationship of beryllium with aluminium.
Or
Beryllium exhibits some similarities with aluminium. Point out three such properties.

Diagonal relationship of beryllium with aluminium:
Since beryllium (Be2+) and aluminium (Al3+) have similar charge/radius ratio, they exhibit diagonal relationship. They resemble as follows:
(i) Both these elements dissolve in strong alkalies to liberate hydrogen and forming beryllate's and aluminates.

(ii) Both have strong tendency to form covalent compounds. Both BeCl2 and AlCl3 are covalent.
(iii) Both form non-volatile, hard oxides (BeO and Al2O3) having very high melting points.
(iv) Both Be and Al form fluoro-complex anions 
(v) Carbides of both the metals react with water liberating methane gas.
Discuss the diagonal realtionship of Be and Al with regard to:
(i) action of alkali (ii) structure of chlorides.
(i) The action of alkali: Both the metals dissolve in strong alkalies to form soluble complexes and liberate hydrogen.

(ii) The structure of chlorides: Structure of BeCl2: In the solid state, it exists as polymeric chain structure. In vapour state, beryllium chloride exists in the dimeric form which decomposes at 1200K into monomeric form. 
The structure of AlCl3: Aluminium chloride exists as a dimer (Al2Cl6). In this dimeric structure, each aluminium atom forms one co-ordinate bond by accepting a lone pair of electrons from the chlorine atoms covalently bonded to the other aluminium atom.
Both the aluminium atoms complete their octet.
Give any three points of similarities between beryllium and aluminium and two points of dissimilarities between beryllium and barium ?
Points of similarities:
(i) BeO as well as Al2O3 are high melting solids.
(ii) Anhydrous chlorides of Be as well as Al are covalent, soluble inorganic solvent and act as Lewis acids.
(iii) Both Be and Al form fluoro-complex anions  and
and  in solution.
in solution.
Points of dissimilarities:
(i) Beryllium does not impart any colour to bunsen flame owing to its small atomic and ionic size in which electrons are held more tightly ; on the other hand, barium imparts apple green colour to the bunsen flame.
(ii) Beryllium is least and barium is most reactive in the group.
How beryllium chloride is prepared? Give its two important properties
Preparation. It is prepared by heating beryllium oxide and carbon mixture in an atmosphere of chlorine. 
Properties:
1. It is covalent in nature and fumes strongly in the moist air due to hydrolysis. 
2. In the vapour phase, it exists as a dimer which dissociates into monomers at about 1200 K.
Name the gases in which magnesium wire continues to burn and write the reactions ?
Mg wire continues to burn in CO2 and SO2 gases. The reactions are:
Draw the structure of: (i) BeCl2 (vapour) (ii) BeCl2 (solid).
In the solid state, BeCl2 has polymeric chain structure. Be atom is tetrahedrally surrounded by four Cl atoms - two are bonded by covalent bonds while the other two by coordinate bonds. The polymeric structure of BeCl2 is due to its electron deficient nature. It has only four electrons in valence shell and can accept two pairs of electrons from neighbouring chlorine atoms to complete their octet.![]()
In the vapour state, beryllium chloride exists as a dimer (Be2Cl4) which dissociates at 1200 K into monomer (BeCl2) which has a linear shape.
How does magnesium occur in nature? How is magnesium obtained by electrolysis method ?
Magnesium docs do not occur in the free state in nature. In the combined state (minerals) it occurs as,
(i) Magnesite MgCO3
(ii) Dolomite MgCO3.CaCO3
(iii) Carnallite KCl MgCl2.6H2O
(iv) Epsom salt MgSO4.7H2O
All plants and animal tissues contain small amounts of magnesium, it is contained in chlorophyll, the green colouring matter of plants. Sea water contains an appreciable amount of magnesium chloride.
Extraction of magnesium: Magnesium is usually extracted by the electrolysis of fused magnesium chloride or carnallite. It is isolated form sea water by Dow process. The various steps employed are
(i) Precipitation of magnesium hydroxide: Sea water is treated with lime water when magnesium hydroxide gets precipitated.
(ii) Conversion of magnesium hydroxide into magnesium chloride: The precipitate of magnesium hydroxide is dissolved in hydrochloric acid to get a clear solution of magnesium chloride.
The solution is concentrated when MgCl2.6H2O crystallises out.
(iii) Preparation of anhydrous magnesium chloride: A current of HCl gas is passed through magnesium chloride hexahydrate when anhydrous magnesium chloride is obtained.
(iv) Electrolysis of anhydrous magnesium chloride: Anhydrous magnesium chloride is added to a molten mixture of sodium chloride and calcium chloride (973 -1023 K). The mixture is then electrolyzed in an electrolytic cell which consists of the iron vessel which acts as a cathode. The anode consists of a graphite rod enclosed in a porcelain hood. The cell is heated externally to about 973 -1073 K. A stream of an inert gas such as coal gas is passed through the cell to check the oxidation of liberated magnesium by atmospheric oxygen. On passing electric current, the following reaction takes place:
At cathode. 
Thus, magnesium is liberated at the cathode while chlorine gas is liberated at the anode.
What happens when:
(i) magnesium is heated with water.
(ii) magnesium is heated in an atmosphere of carbon dioxide,
(iii) magnesium is treated with dilute sulphuric acid and
(iv) magnesium is treated with nitrogen?
(i) Magnesium reacts with boiling water to form magnesium hydroxide and hydrogen. 
(ii) Magnesium burns in the atmosphere of CO2 to form magnesium oxide.

(iii) Magnesium reacts with dilute sulphuric acid liberating dihydrogen gas.

(iv) Magnesium burns in the atmosphere of nitrogen to form magnesium nitride.
Describe briefly the preparation and uses of magnesium chloride (MgCl2.6H2O).
Preparation:
(i) In the laboratory, magnesium chloride is prepared by the action of hydrochloric acid on magnesium oxide or carbonate.

The reaction mixture is cooled and concentrated when crystals of MgCl2.6H2O separate out.
(ii) From carnallite: The mineral carnallite (KCl.MgCl2.6H2O) is powdered and then boiled with water. On cooling KCl is crystallised out while magnesium chloride is left in the mother liquor. The mother liquor is separated, concentrated by evaporation and cooled when crystals of MgCl2.6H2O separate out.
(iii) From sea water: Sea water is concentrated and then treated with lime when magnesium hydroxide gets precipitated.

The precipitate of Mg(OH)2 is dissolved in HCl when a solution of magnesium chloride is obtained. The solution of magnesium chloride on concentration and cooling yields crystals of MgCl2.6H2O.
It is used:
(i) in the preparation of magnesia cement.
(ii) in the extraction of magnesium metal.
How is slaked lime prepared? What are its properties and uses?
Slaked lime is prepared by the following methods:
(i) From quick lime: By treating quicklime with water, slaked lime is formed.

When water is added to quicklime, a huge amount of heat is produced along with the hissing sound.
(ii) From calcium chloride: By treating calcium chloride with caustic soda, slaked lime is formed.
Properties:
(i) Slaked lime is a white amorphous powder.
(ii) A suspension of slaked lime in water is called milk of lime.
(iii) The aqueous layer which is decanted from the precipitated calcium hydroxide is called lime water.
(iv) On passing carbon dioxide through lime water, the lime water turns milky due to the formation of insoluble calcium carbonate. 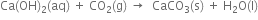
If carbon dioxide is passed in excess, a clear solution is again obtained. This is because the insoluble calcium carbonate changes into soluble calcium bicarbonate.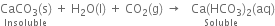
If the clear solution is heated, it again turns milky due to the decomposition of calcium bicarbonate into calcium carbonate. 
(v) Slaked lime reacts with chlorine to form bleaching powder.
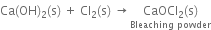
Uses. It is used:
(i) for the preparation of ammonia
(ii) for white washing
(iii) in the purification of sugar
(iv) in the softening of hard water.
What is lime stone? How is it prepared? Give its properties and uses.
Limestone or marble is calcium carbonate (CaCO3).
Preparation: In the laboratory, it is prepared by the action of sodium carbonate on calcium chloride when a white precipitate of calcium carbonate is obtained.
Properties: (i) It is a white powder, sparingly soluble in water.
(ii) On heating to about 1200K, it decomposes to form calcium oxide. 
Uses: It is used
(i) in the form of marble for making floors.
(ii) in toothpaste.
(iii) in the form of fine powder in face powders and
(iv) in the form of limestone for making lime, cement and glass.
How is quick lime prepared on a commercial scale? How is it converted into slaked lime?

Formation of slaked lime: Slaked lime is formed when quicklime is added to water. The reaction is highly exothermic in nature and called slaking of lime.

How will you distinguish between:
(i) Slaked lime (ii) Milk of lime (iii) Lime water?
(i) Slaked lime: It is a white amorphous solid formed when quicklime is added to water.

(ii) Milk of lime: It is the suspension of slaked lime in water.
(iii) Lime water: When milk of lime is kept for some time undisturbed in a beaker and the solution formed on the surface is decanted. It is called lime water.
What is Gypsum? How is it prepared? What are its properties and uses?
Preparation: It can be prepared by the action of dilute sulphuric acid on calcium carbonate or calcium hydroxide.

Properties:
(i) It is a white crystalline solid sparingly soluble in water.
(ii) On heating to 393 K, it changes into


(ii) for manufacturing Plaster of Paris
(iii) as a drying agent.
(iv) for the preparation of ammonia, bleaching powder slaked lime and basic calcium nitrate.
What is Plaster of Paris? How is it prepared? What are its properties and uses?
Plaster of Paris is calcium sulphate hemihydrate.
Preparation:
(i) From gypsum (CaSO4. 2H2O): When gypsum is heated to about 393 - 403 K, partial dehydration takes place with the formation of Plaster of Paris. 
(ii) On a large scale, gypsum is gradually heated in a large steel vessel, holding several tonnes of material. This steel vessel is provided with a mechanical stirrer. During heating, the gypsum is stirred mechanically and the temperature is maintained between 393 - 403 K.
The temperature should be controlled carefully between 393 - 403 K, otherwise above this temperature (say 473 K), the whole of the water of hydration is lost and the gypsum gets dead burnt.
Properties:
(i) It is a white powder.
(ii) When mixed with water, Plaster of Paris quickly solidifies to gypsum with the evolution of heat and also expands slightly.
(iii) The action of heat: When plaster of Paris is heated at 473 K, it forms anhydrous calcium sulphate.
Uses. (i) It is used in surgery for plastering fractured parts of the body.
(ii) It is used for making casts for statues and for preparing blackboard chalks.
What is the effect of heat on the following compounds? (Write equations for the reactions):
(i) Calcium Carbonate
(ii) Magnesium chloride hexahydrate
(iii) Gypsum
(iv) Magnesium sulphate heptahydrate

(ii) MgCl2.6H2O, on heating undergoes hydrolysis by its own water of crystallisation to give magnesium oxide and hyrogen chloride.

(iii) On heating to 390K, it forms a compound having the composition
 which on heating above 473 K, changes into anhydrous calcium sulphate.
which on heating above 473 K, changes into anhydrous calcium sulphate. 
(iv) On heating to 423 K, it changes into monohydrate which on further heating to 473K, forms anhydrous salt.

Anhydrous salt on further heating, forms MgO.

Give one method of preparing quick lime. What happens when rain water falls on it?
Calcium oxide is called quicklime. It is prepared by heating the lime stone in a rotatory kiln at 1070-1270 K.
CO2 formed escapes and hence the above equilibrium shifts towards the formation of calcium oxide.
When rain-water falls on quick lime, slaked lime is formed.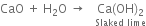
Name a few important uses of quick lime.
It is used:
(i) in the preparation of cement, glass and calcium carbide.
(ii) in the purification of sugar
(iii) in drying alcohol and non-acidic gases like ammonia etc.,
(iv) in softening of hard water.
(v) as a flux in the extraction of metals.
(vi) in agriculture as a fertiliser, disinfectant and germicide.
Describe two important uses of the following;
(a) Caustic soda (b) Sodium carbonate (iii) Quick lime.
(i) Caustic soda
It is used:
(a) in the manufacture of sodium metal, soap (from oils and fats), rayon, paper, dyes and drugs,
(b) for mercerising cotton to make cloth unshrinkable.
(ii) Sodium carbonate
It is used:
(a) in laundries and in softening of water as washing soda,
(b) in the manufacture of glass, caustic soda, soap powders etc.
(iii) Quick lime
It is used:
(a) in the purification of sugar and in the manufacture of dyestuffs,
(b) in the manufacture of bleaching powder, slaked lime and lime colours.
What happens when,
(i) magnesium is burnt in air
(ii) quick- lime is heated with silica
(iii) chlorine reacts with slaked lime
(iv) calcium nitrate is heated?

(ii) Quick- lime on heating with silica (SiO2) gives calcium silicate.
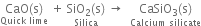
(iii) Chlorine reacts with slaked lime to form calcium hypochlorite.

(iv) Calcium nitrate on heating gives nitrogen dioxide.

Discuss the composition and manufacturing details of cement.
Or
Mention the main constituents of Portland cement.
Cement is essentially a fine grind mixture of calcium aluminates and silicates which set to a hard mass when treated with water.
Composition. The average composition of Portland cement is
CaO 50-60% Fe2O3 1 - 2%
SiO2 20-25% MgO 2 - 3%
Al2O3 5-9% SO3 1 - 2%
Na2O 0.5 - 1%
Manufacturing details: Raw materials. Portland cement is made by fusing together two types of materials:
(i) Calcareous (or rich in lime) such as lime- stone, chalk, alkali waste.
(ii) Argillaceous (or rich in alumina or silica) such as clay, shale, slate etc.
The main raw materials for the manufacture of cement are first crushed separately in a suitable machine. They are then mixed together in required proportions and finely ground (pulverisation). The pulverised mass (either dry or in the formed slurry with water) is introduced into a rotary kiln. The kiln consists of a sheet steel cylinder about 150m in length and 4m in diameter lined inside with fire bricks. It rotates on its axis at the rate of 30-60 revolutions per minute. Due to the rotatory motion, the charge slowly moves down the kiln. A blast of
burning coal dust is blown into the kiln from the lower end. The charge takes 2-3 hours to travel from one end to the other. During its travel, the charge passes through various temperature zones and different reactions take place.
(a) In the upper portion of the kiln (up to 1100K): The material loses its moisture.
(b) In the middle portion (1100 to 1300K). Limestone decomposes to form calcium oxide and carbon dioxide.
(c) At the lower end (1300 - 1800K). Lime and clay combine to form calcium silicate and calcium aluminate.
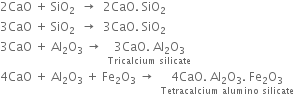
The hard mass thus formed is called clinker. The hot clinkers are cooled and mixed with 2 -3% gypsum and then finally powdered. The powdered material is known as cement and packed in jute or polyethene bags. Gypsum is added to retard the rate of setting of cement. Gypsum reacts with tricalcium aluminate to form calcium sulphoaluminate.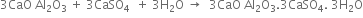
Contrast the action of heat on the following and explain your answer:
(i) Na2CO3 and CaCO3
(ii) MgCl2.6H2O and CaCl2.6H2O
(iii) Ca(NO3)2 and NaNO3.
(i) Na2CO3 and CaCO2:
Na2CO3 does not decompose on heating while CaCO3 decomposes on heating to produce calcium oxide and carbon dioxide.

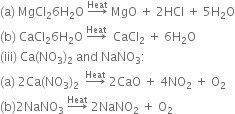
Explain the significance of magnesium and calcium in biological fluids.
Significance of magnesium and calcium:
(i) Magnesium is an important constituent of chlorophyll which initiates the process of photosynthesis in green plants.
(ii) Magnesium ions are concentrated more in intracellular than in extracellular fluids in animal bodies. Their presence is also necessary for the activation of phosphate-transfer enzymes. These enzymes take part in the biochemical process (exothermic) occurring in animal body. Mg2+ ions are also involved in carbohydrates metabolism.
(iii) Ca2+ ions are essential for the formation of bones and teeth. The enamel on teeth is a double salt 3Ca3(PO4)2.CaF2.
(iv) Mg2+ and Ca2+ ions are also involved in the transmission of electrical impulses along the nerve fibre and for the contraction of muscles.
What are the common physical and chemical features of alkali metals?
Physical properties:
i) metallic character: Alkali metals are highly electropositive in nature and hence, they are typical metals. the metallic character is due to low values of ionisation energies and consequently, they have a tendency to lose the valence electrons.
Low ionisation energy: The first ionisation energies of alkali metals are quite low as compared to the elements of the other groups belonging to the same period. the reason is that atoms of alkali metals are of large sizes. Therefore, the outermost electron is far away from the nucleus and can be easily removed. Within the group, ionisation energies of alkali metals decrease as we move down the group.
Low melting and boiling point: The melting and boiling points of alkali metals are very low because of the large size of their atoms due to which inter-particle forces are very weak in them. The melting and boiling points decrease on going down the group(Lithium to Cesium) as the charge density decreases because of the increase in the size of the monovalent cation.
Low electronegativity: Alkali metals have low values of electronegativity. They have very little tendency to attract the shared pair of electrons towards themselves. The electronegativity values of alkali metals decrease as we move down in the group from Li to Cs.
Soft in nature: All the alkali metals are soft and can be cut with the help of a knife.The softness of alkali metals is due to weak metallic bonding in them as the result of the large size of the atoms. As we move down the group, metallic bonding weakens and therefore, softness increases.
Density: Alkali metals have low density due to the large size of metals atoms.
Oxidation state: The alkali metals exhibit oxidation state of +1 in their compounds and strongly electropositive in character. the electropositive character increases from lithium down to caesium in the group.
Chemical properties:
Decomposition of water: The alkali metals decompose water at the ordinary temperature giving out hydrogen.
2Li+2H2O --> 2LiOH +H2
2Na +2H2O --> 2NaOH + H2
2K +2H2O --> 2KOH +H2
Reaction with oxygen: The alkali metals readily burn in oxygen or air to form their oxides
4Li +O2 -->2LiO
combination with halogens: The alkali metals burns in halogens forming their halides
2Na +Cl2 --> 2NaCl
Discuss the general characteristics and gradation in properties of alkaline earth metals.
(i) The general electronic configuration of alkaline earth metals is [noble gas] ns2.
(ii) The atomic and ionic radii of alkaline earth metals are smaller than that of alkali metals. On moving down the group their atomic and ionic radii increase due to the decrease in their effective nuclear charge.
(iii) These metals lose two electrons to acquire the nearest noble gas configuration. Therefore, their oxidation state is +2.
(iv) Due to their small size, the first ionisation enthalpies of alkaline earth metals are higher than those of the alkali metals. But their second ionisation enthalpies are found to be lower than those of the corresponding alkali metals.
v) Metallic character: They are less electropositive than alkali metals due to higher ionisation enthalpies. Metallic character increases down the group due to a decrease in ionisation enthalpy.
Chemical reactivity:
Reaction with water and air: The alkaline earth metals are less reactive than alkali metals. Be and Mg is kinetically inert of O2 and H2O due to the formation of an oxide layer on its surface. Be does not react with water or steam even at red-hot and does not get oxidised in air below 873K. Powdered be burns on ignition to form BeO and Be3N2
2Be +O2 → 2BeO
3Be +N2 → Be3N2
Mg is more electropositive and burns in air with dazzling light forming MgO and Mg3N2.
2Mg +O2 →2MgO
3Mg +N2 →Mg3N2
Ca, Sr and Ba readily react with oxygen to give oxides. Calcium forms oxides whereas Sr and Ba form peroxide. They react with nitrogen to form nitrides.
2Ca +O2→ 2CaO
3Cs +N2 → Ca3N2
Sr +O2 → SrO2
Ba +O2→ BaO2
Mg Reacts with hot water Ca, Ba, Sr react with cold water vigorously.
Mg +H2O(Hot)→ MgO +H2
Ca +2H2O→ Ca(OH)2 +H2
Sr +2H2O → Sr(OH)2 +H2
Ba +2H2O → Ba(OH)2 +H2
Reaction with halogens: Group 2 element react with halogens at increased temperature to from halides.
Be +Cl2+ Heat → BeCl2
Mg +Cl2+ Heat →MgCl2
Ca+Cl2+ Heat →CaCl2
Action with acids: The alkaline earth metals readily react with acids to form salts and liberate H2 gas.
Be +2HCl→ BeCl2+H2
Mg+2HCl→ MgCl2 +H2
Reaction with H2
All metals combine with H2 to form hydrides except Be.
Ca +H → CaH2(Hydrolith)
Mg +H2 → MgH
Compare the alkali metals and alkaline earth metals with respect to (i) ionisation enthalpy (ii) basicity of oxides and (iii) solubility of hydroxides.
|
Alkali metals |
Alkaline earth metals |
|
(i) Ionisation enthalpy: These have the lowest ionisation |
(i) Ionisation enthalpy: Alkaline earth metals have smaller |
|
(ii) Basicity of oxides: The oxides of alkali metals are |
(ii) Basicity of oxides: The oxides of alkaline earth metals are quite basic but not as basic as those of alkali metals. This is because alkaline earth metals are less electropositive than alkali metals. |
|
(iii) The solubility of hydroxides: The hydroxides of alkali metals are more soluble than those of alkaline earth metals. |
(iii) The solubility of hydroxides: The hydroxides of alkaline earth metals are less soluble than those of alkali metals. This is due to the high lattice energies of alkaline earth metals. Their higher charge densities (as compared to |
Explain why can alkali and alkaline earth metals not be obtained by chemical reduction methods?
In the process of chemical reduction, oxides of metals are reduced using a stronger reducing agent. alkali and alkaline earth metals are themselves strong reducing agent and hence cannot be extracted by reduction of their oxides and alkaline earth metals.
alkali metals being highly electron positive cannot be displaced from the aqueous solution of their salts by other metals.
Why are potassium and caesium, rather than lithium used in photoelectric cells?
Compare the solubility and thermal stability of the following compounds of the alkali metals with those of the alkaline earth metals. (a) Nitrates (b) Carbonates (c) Sulphates.
Carbonates of metal: Thermal stability
The carbonates of alkali metals except lithium carbonate are stable to heat. The carbonates of group-2 metals and that of lithium decompose on heating, forming an oxide and carbon dioxide . For example,
Li2CO3 +heat -> Li2O +CO2
MgCO3 +Heat -> MgO +CO2
Na2CO3 +heat -> no effect.
The stabilities of carbonates of alkaline earth metals increase on moving down the group.For example, BeCO3 decompose at 373K, MgCO3 at 813K, CaCO3 at 1173K, SrCO3 at 1563K .
solubilities in water:
carbonates of alkali metals, except Li2CO3, are soluble in water. Their solubilities increase on moving down the group. Carbonates of group-2 metals are almost insoluble in water and their solubilities further decrease on moving down the group. In fact, these metals can precipitate from their salt solutions as carbonates.
Nitrates: Thermal stability
Nitrates of alkali metals,except LiNO3, decompose on strong heating forming nitrites and oxygen. for example,
2KNO3 -> 2KNO2 +O2
Nitrates of alkaline-earth metals and LiNO3 decompose on heating to form oxides, nitrogen to form oxides, nitrogen dioxide and oxygen.
2LiNO3 +Heat -> Li2O +2NO2 +O2
2Ca(NO3)2 +Heat -> 2CaO +4NO2 +O2
Thermal stabilities of nitrates of group-1 and group-2 metals increase on moving down the group from top to bottom.
solubility :
Nitrates of group -1 and group-2 metals are all soluble in water. The solubilities of these salts further increase on descending the group.
Sulphates: Thermal stability
The sulphates of group-1 and group-2 metals are all thermally stable.
solubility:
sulphates of alkali metals are soluble in water. The sulphate of alkaline earth metals is less soluble. Their solubilities decrease on moving down the group from Be to Ba.
Starting with sodium chloride how would you proceed to prepare (i) sodium metal (ii) sodium hydroxide (iii) sodium peroxide (iv) sodium carbonate?
Na is prepared from NaCl by the following method:
2) Sodium hydroxide is prepared by carrying out the electrolysis of the aqueous solution of sodium chloride either in Nelson' cell or Castner Kellner cell.
Preparation of sodium peroxide: Sodium chloride is first converted in sodium by electrolytic reduction. The metals are then heated with an excess of oxygen at about 573K in an atmosphere free from moisture and carbon dioxide to form sodium peroxide.
3) sodium carbonate is prepared from an aqueous solution of NaCl by Solvay process. In this process, CO2 is passed through NaCl solution saturated with ammonia when the following reaction occurs :
:
Describe the importance of the following: (i) limestone (ii) cement (iii) plaster of paris.
Importance of limestone
It is used in the manufacture of quick lime.
CaCO3 --> CaO +CO2
Quicklime, when added to water in limited amount, is used to produce slaked lime or milk of lime Ca(OH)2
CaO +H2O ---> Ca(OH)2
It is used as a flux to remove acidic impurities
CaO +SiO2 ---> CaSiO3
6CaO + P4O10--> 2Ca3(PO4)2
The importance of cement: Cement is an important building material. it is used in the concrete and reinforced concrete, in plastering and in the construction of dams, bridges and buildings.
The importance of plaster of Paris:On heating gypsum at 373 K, it loses water molecules and becomes calcium sulphate hemihydrate plaster of Paris.Plaster of Paris is a white powder and on mixing with water, it changes to gypsum once again giving a hard solid mass. It is used for immobilising the affected part where there is a bone fracture. It is used for making statues, models and other decorative materials.
Which one of the following alkaline earth metal sulphates has its hydration enthalpy greater than its lattice enthalpy?
-
CaSO4
-
BeSO4
-
BaSO4
-
SrSO4
B.
BeSO4
Hydration energy basically depends on the size of an atom.
(i) Smaller the size atom greater the hydration energy.
(ii) Be is small in size thus have greater hydration energy.
The correct statement for the molecule, CsI3 is
-
It is a covalent molecules
-
It contains Cs+ and I3-
-
It contains Cs3+ and I- ions
-
It contains Cs+, I- and lattice I2 molecule
B.
It contains Cs+ and I3-
I3- is an ion made up of I2 and I- which has linear shape
While Cs+ is an alkali metal cation.
Both lithium and magnesium display several similar properties due to the diagonal relationship; however, the one which is incorrect is :
-
Both form basic carbonates
-
Both form soluble bicarbonates
-
Both form nitrides
-
Nitrates of both Li and Mg yield NO2 and O2 on heating
A.
Both form basic carbonates
Lithium can form carbonate (Li2CO3). Lithium Carbonate is not basic in nature. On other hands, Mg can form basic carbonate.
5Mg+2 + 6CO32- + 7H2O → 4 MgCO3.Mg(OH)2.5H2O + 2HCO3-
Which one of the alkali metals, forms only the normal oxide, M2O on heating in air?
-
Rb
-
K
-
Li
-
Na
C.
Li
Li is the alkali metal which forms only normal oxide Li2O when heated in air.
2Li +1/2 O2 ---> Li2O
sodium, when heated in air forms peroxide while heavier alkali metals form superoxide as the major product.
In the replacement reaction![]()
The reaction will be most favourable if M happens to be
-
Na
-
K
-
Rb
-
Li
C.
Rb
For the reaction,![]()
The reaction will be faster with Rb because lattice energy of RbF is less than LiF, NaF, KF.
Which of the following compounds has the lowest melting point?
-
CaBr2
-
CaI2
-
CaF2
-
CaCl2
B.
CaI2
As covalent character increases, melting point decreases, thus order of melting point is
CaF2 > CaCl2 > CaBr2 > CaI2
Which one of the following is present as an active ingredient in bleaching powder for bleaching action?
-
Ca(OCl)2
-
CaO2Cl
-
CaCl2
-
CaOCl2
D.
CaOCl2
CaOCl2, Calcium hypochlorite is the active ingredient in bleaching powder which releases chlorine.
On heating which of the following releases CO2 most easily?
-
K2CO3
-
Na2CO3
-
MgCO3
-
CaCO3
C.
MgCO3
Order of thermal stability is
K2CO3 > Na2CO3 > CaCO3 > MgCO3
Thus, MgCO3 releases CO2 most easily
MgCO3 + heat ---> MgO + CO2
What is the value of electron gain enthalpy of Na+ if IE1 of Na = 5.1 eV?
-
-5.1 eV
-
-10.2 eV
-
+2.55 eV
-
+10.2 eV
A.
-5.1 eV
IE1 of Na = - Electron gain enthalpy of Na+ ion.
= -5.1 eV.
Match (I) with list (II) for the compositions of substances and select the correct answer using the code given below the lists.
|
|
List I (substance) |
|
List II Composition |
|
A. |
Plaster of Paris |
1. |
CaSO4.2H2O |
|
B. |
Epsomite |
2. |
CaSO4. ½ H2O |
|
C. |
Kieserite |
3. |
MgSO4. 7H2O |
|
D. |
Gypsum |
4. 5. |
MgSO4.H2O CaSO4 |
-
A
B
C
D
3
4
1
2
-
A
B
C
D
2
3
4
1
-
A
B
C
D
1
2
3
5
-
A
B
C
D
4
3 2 1
B.
|
A |
B |
C |
D |
|
2 |
3 |
4 |
1 |
Plaster of paris = CaSO4.1/2 H2O
Epsomite =MgSO4.7H2O
Gypsum = CaSO4.2H2O
Kieserite = MgSO4.H2O
Property of the alkaline earth metals that increases with their atomic number
-
the solubility of their hydroxides in water
-
the solubility of their sulphates in water
-
ionisation energy
-
electronegativity
A.
the solubility of their hydroxides in water
Electronegativity, as well as ionisation energy both, usually decrease on moving downward a group with an increase in atomic number. The hydroxides and sulphates of alkaline earth metals are ionic solids and the solubility of ionic solids is governed by two factors viz, lattice energy and hydration energy. For solubility hydration, energy > lattice energy.
Hydration energy varies inversely with size, ie, decreases with increase in size. However, lattice energy in case of sulphates, remains almost same with an increase in the atomic number of alkaline earth metals, due to the large size of sulphates ion. Hence, hydration energy only governs the solubility of alkaline earth metal sulphates decrease as the hydration energy decreases on moving downward the II A group.
Which of the following alkaline earth metal sulphates has hydration enthalpy higher than the lattice enthalpy?
-
CaSO4
-
BeSO4
-
BaSO4
-
SrSO4
B.
BeSO4
Hydration energy varies inversely with the size and in sulphates of alkaline earth metals lattice energy remains almost constant.
The order of size of alkaline earth metal is
Be2+ > Ca2+ > Sr2+ > Ba2+
Hence, BaSO4 has the hydration enthalpy higher than the lattice enthalpy.
In the case of alkali metals, the covalent character decreases in the order
-
MCl> MI>MBr>MF
-
MF> MCl> MBr>MI
-
MF > MCl> MI>MBr
-
MI>MBr>MCl>MF
D.
MI>MBr>MCl>MF
According to Fajan's rule
In the given options, the cation is same but anions are different. Among halogens the order of size is
F < Cl< Br< I
therefore, Order of covalent character is
MI> MBr>MCl>MF
The alkali metals form salt-like hydrides by the direct synthesis at elevated temperature. The thermal stability of these hydrieds decreases in which of the following orders?
-
CsH > RbH > KH > NaH >LiH
-
KH > NaH> LiH > CsH >RbH
-
NaH > LiH >KH> RbH> CsH
-
LiH > NaH > KH >RbH >CsH
D.
LiH > NaH > KH >RbH >CsH
Small anion forms stable compounds with small cation.
The thermal stability of alkali metal hydrides decreases as:
LiH> NaH > KH > RbH >CsH
because the size of cation increases as;
Li+ < Na+ < K+ < Rb+ <Cs+
The correct order of increasing thermal stability of K2CO3, MgCO3, CaCO3 and BeCO3 is:
-
BeCO3 < MgCO3 < K2CO3 <CaCO3
-
BeCO3 < MgCO3 < CaCO3 <K2CO3
-
MgCO3 < BeCO3 < CaCO3 < K2CO3
-
K2CO3 < MgCO3 < CaCO3 < BeCO3
B.
BeCO3 < MgCO3 < CaCO3 <K2CO3
Thermal stability of carbonates increases in a group as we move from top to bottom and decreases in a period as we move from left to right. so, the correct order of thermal stability of given carbonates is:
BeCO3 < MgCO3 < CaCO3 < K2CO3
Be, Mg and Ca present in second group and K present in the first group.
In which of the following the hydration energy is higher than the lattice energy?
-
BaSO4
-
MgSO4
-
RaSO4
-
SrSO4
B.
MgSO4
Hydration energy of sulphate decreases from top to bottom in an IInd group. Mg2+ is smaller than other ions of that group so Mg2+ is readily hydrated. MgSO4 has higher hydration energy than lattice energy.
The correct order of the mobility of the alkali metal ions in aqueous solution is:
-
Li+>Na+ > K+ > Rb+
-
Na+>K+ > Rb+ > Li+
-
K+ > Rb+ >Na+ > Li+
-
Rb+ > K+ > Na+ > Li+
D.
Rb+ > K+ > Na+ > Li+
The correct order of the mobility of the alkali metal ions in aqueous solutions
Rb+ > K+ > Na+ > Li+ due to following order of hydration energy of these ions Li+ > Na+ > K+ > Rb+ and due to hydration of ion, mobility decreases.
Which of the following oxides is most acidic in nature?
MgO
BeO
CaO
BaO
B.
BeO
In metals moving down the group metallic character increases, so basic nature increases hence most acidic will be BeO.
Among CaH2, BeH2, BaH2, the order of ionic character is
BeH2< CaH2<BaH2
CaH2<BeH2<BaH2
BaH2<BeH2<CaH2
BeH2 < BaH2 < CaH2
A.
BeH2< CaH2<BaH2
Smaller the size cation, more will be its polarising power. Hence, BeH2 will be least ionic.
Or
On moving down the group metallic character of metals increases so ionic character of metal hydride increases.
Which of the following is the correct order of stability for the given superoxides?
KO2 < RbO2 < CsO2
CsO2 < RbO2<KO2
RbO2
KO2 < CsO2 <RbO2
A.
KO2 < RbO2 < CsO2
With a progressive increase in the size of alkali metal ions, the stability of superoxides increases because the size of superoxide ion is large and large cation can be stabilised more by large anion.
Therefore, the order of stability is
KO2 <RbO2 < CsO2
Mock Test Series
Sponsor Area
Sponsor Area