Chemistry I Chapter 7 The P-Block Elements
Sponsor Area
NCERT Solution For Class 12 Business+studies Chemistry I
Why are pentahalides more covalent than trihalides?
Why is BiH3 the strongest reducing agent amongst all the hydrides of Group 15 elements?
Why is N2 less reactive at room temperature?
Mention the conditions required to maximise the yield of ammonia.
Ammonia is prepared using the Haber’s process. The yield of ammonia can be maximized under the following conditions:
(i) Temperature ~ 700k.
(ii) High pressure of 200 x 105 Pa (about 200 atm).
(iii) A catalyst such as iron oxide with small amounts of K2,O and Al2O3.
How does ammonia react with a solution of Cu2+?
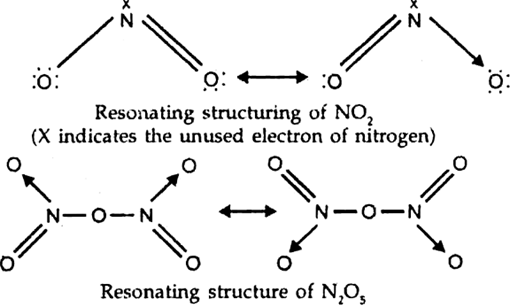
What is the covalency of nitrogen in N2O5?

From the structure of N2O5’ it is evident that covalency of nitrogen is four.
Bond angle in PH4+ is higher than that in PH3. Why?

What happens white phosphorus is heated with NaOH solution in an inert atmosphere of CO2?
What happens when PCl5 is heated?
Write a balanced equation for the hydrolytic reactions PCl5 in heavy water.
POCl3 + 3 D2O → D3PO4 +3DCl
therefore the net reaction can be written as:
PCl5 +4D2O → D3PO4 +5DCl
What is the basicity of H3PO4?

What happens when H3PO3 is heated?
H3PO3 on heating disproportionates to give orthophosphoric acid and phosphine.
4H3 PO3→3H3PO4 + PH3
List the important source of sulphur.
Write the order of thermal stability of the hydrides of Group 16 elements.
H2O>H2S>H2Se>H2Te>H2PO
Why is H2O a liquid and H2S a gas?

structure of water molecule.
Which of the following does not react with oxygen directly?
Zn, Ti, Pt, Fe
Complete the following reactions
(i) C2H4 + O2 →
(ii) 4Al + 3O2 →
i) C2H4 + O2 → 2CO2 + 2H2O
Al combines with O2 to form alumina.
(ii) 4Al + 3O2→ 2Al2O3
Sponsor Area
Why does O3 act as a powerful oxidising agent?
O3 → O2 +O (nascent oxygen)
since nascent oxygen is very reactive therefore O act as powerful oxidising agent.
How is O3 estimated quantitatively?
2I-(aq) +H2O(l) +O3(g) → 2OH-(aq) +I2(s) +H2O(g)
Iodine is liberated which can be titrated against a standard solution of sodium thiosulphate. This is a quantitative method of estimating the O3 gas.
What happens when sulphur dioxide is passed into aqueous solution of Fe (III) salt?
SO2 reduces Fe (III) salt to iron (II) salt.
2Fe3 + + SO2, + 2H2 O→ 2Fe2+ + SO4,2' + 4H+
Comment on the nature of two S-O bonds formed in SO2 molecule. Are the two S-O bonds in this molecule equal?
How is the presence of SO2 detected?
SO2 decolourises acidifed potassium permanganate (VII) solution.
5SO2+ 2MnO–4, + 2H2O→ 5SO4 2– + 4H+ + 2Mn2+
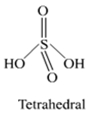
Mention three areas in which H2SO4 plays an important role.
H2SO4 plays an important role.
(i) In the manufacture of fertilizers.
(ii) In detergent industries.
(iii) In storage batteries.
Write the conditions to maximise the yield of H2SO4 by contact process.
Conditions are:
(i) High concentration of reactants.
(ii) Low temperature. But an optimum temperature of 623-723 k must be maintained.
(iii) High pressure: Normally a pressure of about two atmosphere is maintained.
(iv) Presence of catalyst: In order to accelerate the reaction, the presence of catalyst is quite helpful. V2O5, is used as a catalyst.
(v) Purity of gases: Gases must be completely free from dust and poisonous gases like arsenic oxide before they are passed through the catalyst.
Why is Ka2 < < Ka, for H2SO4 in water?
In aqueous solution, sulphuric acid ionizes in two steps:
H2 SO4 is very strong in water largely because of its first ionisation to H3O+ and HSO4–.
H2SO4(aq) +H2O(l)→ H3O+ (aq) + HSO4- (Ka1
HSO4- + H2O(l) → H3O+ +SO4-2 (Ka2
The ionisation of HSO4– to H3O– and SO42– is very small. That is why Ka2 < < Ka1,.
Considering the parameters such as bond dissociation enthalpy, electron gain enthalphy and hydration enthalpy compare the oxidising power of F2 and Cl2.
Give two examples to show the anomalous behaviour of fluorine.
(i) Oxidation state: Fluorine shows oxidation state of – 1 only. It does not show any positive oxidation state. Other halogens show oxidation states such as + 1, + 3, + 5, + 7 also.
(ii) Extra-ordinary reactivity: Fluorine is extraordinary most reactive element. This is due to F—F bond energy is very low as compared to that of other halogen molecules.
Sea is the greatest source of some halogens. Comment.
Give the reason for bleaching action of Cl2.
Cl2 liberates nascent oxygen in presence of moisture or in aqueous solution
Cl2 + H2O→ 2HCl + O
Colourled substance + O → Colourless substance
This nascent oxygen bleaches the coloured substance present in vegteable substance due to oxidation.
Name two poisonous gases which can be prepared from chlorine gas.
Why is ICl more reactive than I2 ?
Why is helium used in dividing apparatus?
Why has it been difficult to study the chemistry of radon?
Though nitrogen exhibits + 5 oxidation state it does not form pentahalide. Give reasons.
PH3 has lowerboiling point than NH3. Why?
Write the reaction of thermal decomposition of sodium azide.
Thermal decomposition of sodium azide gives dinitrogen gas.
2NaN3 → 2Na + 3N2
Why does NH3 act as a Lewis base?
Why does NO2 dimerise?
Sponsor Area
What type of hybridisation is associated with N in NH3,? What is the expected bond angle in NH3?
In the ammonia molecule (NH3), 2s and 2p orbitals create four sp3 hybrid orbital, one of which is occupied by a lone pair of electrons
sp3 hybridisation, expected bond angle = 109°28', actual bond angle = 107°.

NO2 is coloured but its dimer N2O4 is colourless. Why?
Arrange the hydrides of group 15 in decreasing order of basic strength.
The correct order is
Why ammonia is a good complexing agent?
The experimentally determined N — F bond length in NF3 is greater than the sum of the single bond covalent radii of N and F. Why?
In what why it can be proved that PH3 is basic in nature?
PH3 reacts with acids like HI to form PH4I which shows that it is basic in nature.
PH3, + HI → PH4I
Due to lone pair on phosphorus atom, PH3 is acting as a Lewis base in the above reaction.
Nitric acid (dilute or conc) renders aluminium passive. Why?
Phosphine has a lower boiling point than ammonia. Give a reason.
On being slowly passed through water PH3 forms bubbles but NH3 dissolves. Why is it so?
Nitrogen does not form any pentahalides. Give reason.
Nitrogen with n = 2, has s and p orbitals only. It does not have d orbitals to expand its covalence beyond four. That is why it does not form pentahalide.
Phosphorus does not form P2 molecular structure. Why?
Why does PCl3 fume in moisture?
PCl3 + 3H2O→ H3PO3 + 3HCl
Are all the five bonds in PCl5 molecule equivalent? Justify your answer.
How do you account for the reducing behaviour of H3 PO2 on the basis of its structure?
Why is H3PO3diprotic?
What are flowers of phosphorus?
H2S is less acidic than H2Te. Why?
H2S is less acidic than H2Te because as we movedown the group,the bond dissociation enthalpy decreases i.e it becomes easy to dissociate or
break the bonds(due to the increasing size down the group).So,with this concept in terms of hydrides,it becomes easy for H2Te to remove H easy
as H+ because of its low bond dissociation enthalpy(as mentioned before).Therefore , H2Te is
more acidic.
What is the difference between the nature of -bonds present in H3PO3 and HNO3?
The tendency to show-2 oxidation state diminishes from S to Po. Why?
Why does oxygen not show an oxidation state of + 4 and + 6 like sulphur?
The electronic configuration of oxygen is 1s2 2s2 2px2 2py12pz1 i.e. it has two half-filled orbital and there is no d-orbital available for excitation of electron. Further, it is the most electronegative element of its family. Hence it shows oxidation state -2 only. Other elements like sulphur have d-orbital available for excitation, thereby giving four and six half-filled orbitals; moreover they can combine with more electronegative elements. Hence they shows oxidation states of +2,+4 and +6 also.
Give one example each of the oxoacids of phosphorus in which the P has the oxidation state of (i) + 4 (ii) + 3.
Name all the elements of group 16. Which elements is radioactive?
Polonium is a radioactive element. .
Why are group 16 elements called chalcogens?
Oxygen is the most abundant of all the elements on earth. Oxygen forms about 46.6% by mass of earth’s crust. Dry air contains 20.946% oxygen by volume.
Give two important uses of Selenium.
(i) In photocopier machines as photo– conductor.
(ii) As the decolouriser of glass.
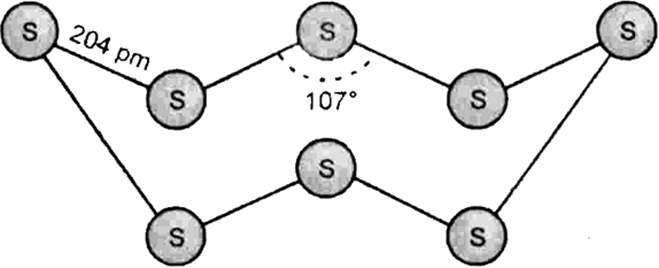
Name two allotrope of sulphur. Which allotrope is stable at room temperature?
Two allotropes of sulphur are:
(i) Yellow ortho-rhombicα and
(ii) β-monoclinic
Ortho rhombic allotrope of sulphur is stable at room temperature.
Why does sulphur in vapour state exhibit paramagnetic behaviour?
Why are group 16 elements called polymorphic elements?
Why oxygen has less tendency to catenate than sulphur?
Oxygen is small in size and the lone pair on oxygen repel the bond pairs of O-O bond to larger extent than the lone pairs on sulphur in S-S bond.
The S-S bond energy 213K/J mol therefore bond strength is more compared to O-O bond energy 138k/J mol. Sulphur naturally exist in nature as S8 molecules. On heating these rings break and link jointly in long to chains. Hence sulphur has great tendency for catenation than oxygen.
Write the name of the compound which is formed by Krypton.
Arrange the hydrides of group 16 in order of increasing boiling point.
H2O<H2S < H2Se< H2Te
Arrange hydrides of group 16 in order of increasing acidic strength.
Electron density on central atom decreases and consequently its tendency to donate a pair of electron decreases and hence its Acid strength increase.
The correct order is
H2O < H2S < H2Se < H2Te.
Give the preparation of sulphur tetrafluoride.
Why is SF6 chemically inert?
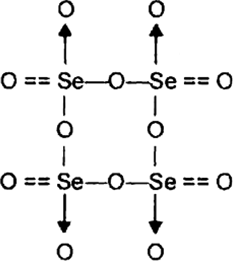
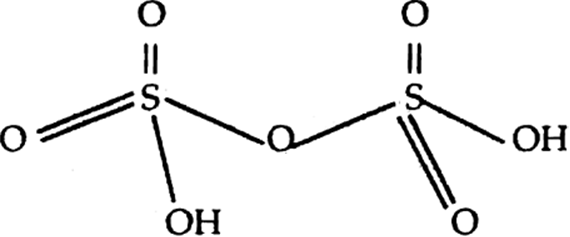
How manv S—S linkages present in sulphur trioxide trimer, S3O9?
Why is sulphuric acid a good dehydrating agent?
What happens when conc. H2SO4 is slowly added to cane sugar?
When conc. H2SO4 is added to cane sugar then charring of sugar takes place:
C12H22O11(s) + H2SO4(aq) + 1/2 O2(g) → 11C(s) + CO2(g) + 12H2O(g) + SO2(g)
Sponsor Area
Which form of sulphur shows paramagnetic behaviour?
S2 molecule has two unpaired electrons in the antibonding orbital hence, exhibits paramagnetism.
What happens when
(i) Concentrated H2SO4 is added to calcium fluoride
(ii) SO3 is passed through water?
(i)When conc. H2SO4 is added to calcium fluoride,It forms hydrogen fluoride and calcium sulphate.
CaF2 + H2SO4→ CaSO4 + 2HF
(ii)When SO3 is dissolves in water. It produce Sulphuric acid(H2SO4)
SO3 + H2O→ H2SO4
Why ozone is more reactive than oxygen?
O3 -----> O2 +O(nascent oxygen)
Sulphur melts to clear mobile liquid at 119°C, but on further heating above 160°C, it becomes viscous. Why?
The wooden shelf under the reagent bottle containing concentrated H2SO4 blacken after somtime. Why?
Although electron gain enthalpy of fluorine is less negative as compared to chlorine, fluorine is a stronger oxidising agent than chlorine. Why?
Oxidizing agents are substances that gain electrons in a chemical reaction
Fluorine is a stronger oxidising agent than chlorine because:
(i) Low enthalpy of dissociation of F—F bond.
(ii) High hydration enthalpy of F–
Arrange the following in the decreasing order of bond energy; F2, Cl2, Br2 I2.
Why fluorine has low electron gain enthalpy than chlorine?
Why fluorine shows an oxidation state of – 1 only?
Arrange the following in order of decreasing property indicated:
(a) M—F, M—Cl, M—Brand M— l. (Ionic character)
(b) F2,Cl2,Br2,I2. (reactivity)
(a) M—F > M—Cl > M—Br > M—I.
(b) F2 > Cl2 > Br2 > I2.
because, as we move down the group: The atoms get larger. The outer shell gets further from the nucleus. The attraction between the nucleus and electrons gets weaker, so an electron is less easily gained.
Fluorine can not be prepared from fluorides by chemical treatment. Why?
1 /2 F2 + e→ F – E° = maximum
Further more, electrolysis can liberate F2, but F2 being very reactive, is again reduced to F– ion the most stable ion.
Name two compounds in which halogens exhibit + ve oxidation states.
Br2O7, BrF3.
Which halogen does not show positive oxidation state?
Arrange the halogens in order of their m.pt. and b.pt.
F2 < Cl2 < Br2 < I2 < At2.
Which halogen will produce O2 and O3, on passing through water?
2 F2 + 2 H2O ---> 4HF + O2
OF2 is called oxygen difluoride rather than fluorine oxide, why?
Write the balanced chemical equation for the reaction of Cl2 with hot and concentrated NaOH. Is this reaction is redox reaction? Justify.
Yes, chlorine from zero oxidation state is changed to – 1 and + 5 oxidation states.
How will you prepare hyrogen iodide?
2I2 + N2H4 → N2 + 4HI
HI can be prepared by simply combining H2 and I2:
H2 + I2 → 2 HI
Why HF has abnormally high melting and boiling points?
HF can not be stored in glass bottles. What is the reason for this?
What happens when sulphur dioxide is passed through an acidic solution of sodium chlorate?
2NaClO3 + SO2→ 2ClO2+ Na2SO4
Oxygen is sp3 hybridised in both H2O and Cl2O, but in H2O, the bond angle is 104° while in Cl2O, the bond angle is 112°. Account for this difference in the value of bond angle.
Give two uses of chlorine dioxide (ClO2).
(i) It is used as a bleaching agent for paper pulp and textiles.
(ii) It is used as disinfectant in sewage and drinking water.
Explain why fluorine form only one oxoacid, HOF.
This is due to .
(i) small size and high electronegativity of fluorine
(ii) non-avilability of d-atomic acid.
How will you prepare pure hypo-chlorus acid?
2Cl2 + H2O + 2HgO→ HgO.HgCl2 + 2HOCl
Arrange the following in order of decreasing basic strength:
(a) HCIO, HCIO2, HClO3, HClO4
(b) HOCl, HOBr, HOI
(a) HClO4> HClO3> HClO2 > HCIO
(b) HOCl > HOBr > HOI
What are interhalogen compounds? Give two examples.
Most interhalogen compounds known are binary.
Examples are ClF3’ IF5.
Write the equation for the preparation of ClF from uranium.
U + 3ClF3→ UF6 + 3ClF.
What happens when iodine is added to potassium iodide solution?
Iodine is not very soluble in water, therefore the iodine reagent is made by dissolving iodine in water in the presence of potassium iodide. This makes a linear triiodide ion complex with is soluble. The triiodide ion ion slips into the coil of the starch causing an intense blue-black colour.
KI → K+ + I-
I2 + I- → I3-
Thus the reaction of
I2 + 2KI → KI + KI3
Give two examples of poly halonium cation
Example of poly halonium cation.
(i) Cl F2+ (ii) IC12+.
HCl when reacts with finely powdered iron, forms ferrous chloride and not ferric chloride. Why?
Its reaction with iron produces H2
Fe + 2HCl→ FeCl2 + H2
Liberation of hydrogen prevents the formation on of ferric chloride.
Hydrogen halides are covalent compounds but their aqueous solutions conduct electricity, Why?
Hydrogen halides are covalent compound but in aqueous phase conduct electricity because of the formation of H3O+ and X– ions.
HX + H2O→ H3O+ + X–
Why chlorine displaces iodine from potassium salts?
Cl2(aq) + 2KI(aq) ==> 2KCl(aq) + I2(aq)
When blue litmus is dipped into a solution of hypochlorous acid, it first turns red and is then decolorized. Why?
Formation of HCl change the colour of litmus paper.
Bleaching of flowers by Cl2 is permanent, while by SO2 it is temporary. Give reason.
When Chlorine react with water it form nascent oxygen which act as oxidising agent.
Cl2 +H2O -----> 2HCl +[O]
no such oxidising agent is shown in case of SO2
SO2 + H2O ---->H2SO3
Hence product bleached by SO2 is reoxidised by air to its original form.
Bleaching powder loses its bleaching property when kept in an open bottle for a long time. Give reason.
Chlorine is responsible for the bleaching property.
when bleaching powder bottle kept for a long time it react with CO2 and produce chlorine.
henceloses the property of bleaching.
The brown colour of an acidified dilute solution of iodine in aqueous potassium iodide is intensified by the addition of a nitrite but is discharged by the addition of sulphite.
2KI + HNO3 ----> 2KNO2 +2H2O +2NO +I2
Thus more iodine is produced from KI and colour is intensified.
The sulphrous acid H2SO3 is a reducing acid
H2SO3 ---> H2O + SO2
SO2+ 2H2O ------> SO42- +4H+ +2e-
I2 +2e ----> 2I-
Due to conversion of iodine iodine (brown ) in iodine I- colourless.
With what neutral molecule is CIO– iso– electronic?
valence electron of CIO–. is 14= 7+6+1(negtive charge)
valence electron of ClF is 14 =7+7
ClF is isoelectronic with ClO–.
Sponsor Area
Why is OF6 compound not known?
Noble gases have very low boiling points. Why?
Name the first compound of noble gases. Who isolated this compound?
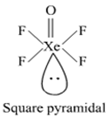
Give an equation in which the Xenon fluoride act as a
(i) fluoride donor (ii) fluoride acceptor.
(i) XeF2, + PF5 - → [XeF]+ [PF6]–
Here fluroide act as donar.
(ii) XeF6 + NaF → Na+ [XeF7]–
Here fluroide act as acceptor.
What happens when Xenon hexafluoride is being hydrolysed completely?
when Xenon hexafluoride is hydrolysed XeO3 is formed.
XeF6 + 3H2O → XeO3 + 6HF
How will you prepare Xenon oxy-tetrafluoride?
Xenon oxytetra fluoride can be prepared by the partial hydrolysis of XeF6.
XeF6 + H2O→ XeOF4 + 2HF
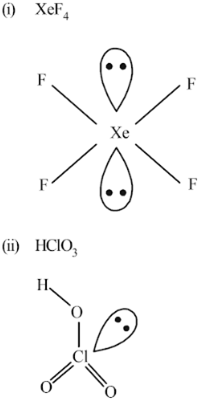
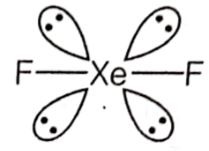
Give the formula and describe the structure of a noble gas species which is isostructural with:
(a) ICl4–, (b) IBr2–, (c) BrO3–
(a) ICl4– = XeF4
(b) IBr2– =XeF2
(c) BrO3–=XeO3
Give two uses of argon.
Argon is inert gas.
(i) It is used to provide an inert atmosphere in welding of metals and alloys.
(ii) In electric bulbsas a inert gas.
Does the hydrolysis of XeF6 lead to a redox reaction?
XeF6 + H2O --->XeOF4 +2HF
XeO+ H2O ---> XeO2F2 +2HF
XeO2F2 + H2O----> XeO3 +2HF
Hence the XeF6 does not show redox reaction.
Why does R3P = O exist but R3N = 0 does not (R = alkyl group)?
On other hand P due to having d- oribital forms mutiple bonds and hence can expand covalency beyond 4.
thus R3P = 0 exists but R2 N = 0 does not exist.
Give the disproportionation reaction of H3 PO3.
4H3PO3 → 3H3PO4 + PH3
How is SO2 an air pollutant?
Why are halogens strong oxidising agents?
Arrange the following in the order of increasing bond enthalpy: F2’ Cl2 Br2, l2.
I2 < Br2, < F2 < Cl2.
Why are halogens coloured?
Explain why inspite of nearly the same electronegativity, oxygen forms hydrogen bonding while chlorine does not.
Write the reactions of F2 and Cl2 with water.
Fluorine reacts violently with water forming hydrogen fluoride, and liberates oxygen which is highly charged with ozone.
(i) 2F2(g) + 2H2O(l) → 4H+ (aq) + 4F–(aq) + O2(g)
Chlorine dissolves in water to some extent to give a green solution
(ii) Cl2+ H2O → HCI + HOCl
Why only F and O form the compounds with Xe?
Arrange hypophalous acids in the order of decreasing acidic nature.
HCIO > HBrO > HI.
What happens when XeF6 is hydrolysed?
When Xenon is hydrolysed it form trioxide.
XeF6 + 3H2O→ XeO3 + 6HCl.
Give an example of a compound in which the oxidation state of chlorine atom is + 7.
What kind of bond is expected between oxygen and fluorine in oxygen fluoride?
Predict the shape of ClF3 on the basis of VSEPR theory.

In which one of the two structures, NO2– and NO2–, the bond angle has a higher value?
This is because in NO2– there is extra lone pair of electron present and thus lone pair - lone pair repulsion and thus decrease in bond angle.
Which is a stronger acid in aqueous solutions, HF or HCl?
HCl is a stronger acid in aqueous solution.because F is small in size and thus have stronger hydrogen bond than Cl. acid chracter of HCl is more than HF. it is easily dissociate to give H+ ion in solution
Why is the bond angle in PH3 molecule lesser than that in NH3 molecule?
Why does the reactivity of nitrogen differ from phosphorus?
Why does NH3 form hydrogen bond but PH3 does not?
N—H bond is reasonably polar and this leads to hydrogen bonding. As the bond polarity of the P—H bond is almost negligible, PH3is not involved in hydrogen bonding.
How is nitrogen prepared in the laboratory? Write the chemical equations of the reactions involved.
How is ammonia manufactured industrially?
A mixture of dry nitrogen and hydrogen gases in the ratio of 1:3 by volume is compressed to about 200 to 300 atm and passed over iron catalyst at a temperature of about 723 k to 773 k. The iron catalyst is mixed with aluminium oxide (Al2O3) and potassium oxide (K2O) which act as promotors.
Ammonia being formed is continuously removed by liquefying it.
The optimum conditions for the production of ammonia are a pressure of about 200 atm, a temperature of ~700 k and use of a catalyst such as iron oxide with small amounts of K2O and Al2,O3 to increase the rate of attainment of equilibrium.

Illustrate how copper metal can give different products with HNO3.
The product of oxidation depend on the concentration of acid, temperture and also the material undergoing oxidation.
Two condition aries :
On heating with dilute nitric oxide NO evolved or on reacting with concentrated nitric acid NO2 is evolved.
The HNH angle value is higher than HPH, HAsH and HSbH angles. Why?
The difference in the bond angles is based on the electronegativity and the size of the central atom. For example, nitrogen is the smallest in size with maximum elctronegativity (3.0). The electron density is very hgh around nitrogen which also means strong repulsions in the electron pairs around it resulting in maximum bond angle (107°). As we move down the group, the atomic sizes increase and the electronegativities decrease. As a result, there is a gradual decrease in the electron density on the central atom resulting in decreased bond angles in the same order. Thus, the HNH angle value is higher than HPH, HAs H and HSbH angles.
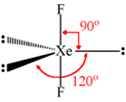
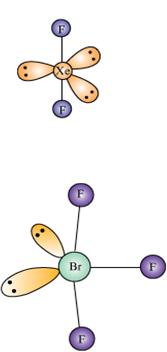
Describe the molecular shape of BrF3 on the basis of VSEPR theory.
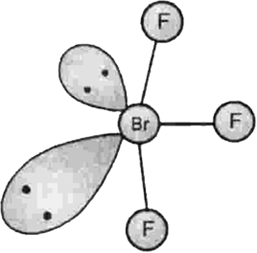
Fig. Molecular shape of BrF3
According to VSEPR theory, these will occupy the corners of a trigonal bipyramid. The two lone pairs will occupy the equatorial positions to minimise lone pair-lone pair and the bond pair-lone pair repulsions which are greater than the bond pair-bond pair repulsions. In addition, the axial fluorine atoms will be bent towards the equatorial fluorine in the order to minimise the lone pair-lone pair repulsions. The shape would be that of a slightly bent‘T’.
Explain why NH3 is basic while BiH3 only feebly basic.
Nitrogen atom has the smallest size and a very high electron density aroun the nitrogen. Therefore, its electron releasing tendency or the basic strength is the maximum. Down the group, there is a gradual increase in atomic size and decrease in the electron density on the central atom. Consequently, the electron releasing tendency or basic strengths of the hydrides decrease in the order as given below
NH3 > PH3 > AsH3 > Sb H3 > BiH3
Therefore, NH3 is basic while BiH3 is only feebly basic.
Nitrogn exists as diatomic molecule but phosphorus as P4, Why?
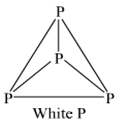
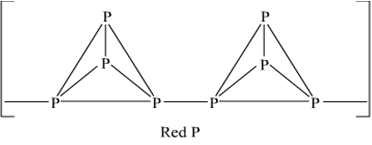
Write main differences between the properties of white phosphorus and red phosphorus.
|
Properties |
White Phosphorus |
Red phosphorus |
|
1. Colour |
White but turns |
Dark red |
|
yellow on exposure |
||
|
2. State |
Waxy solid |
Brittle powder |
|
3. Density |
1.84 g cm-3 |
2.1 gcm3 |
|
4. Ignition |
307 K |
543 K |
|
temperature |
||
|
5. Stability |
Less stable at ordi- |
More stable at ordi – |
|
nary temperature. |
nary temperature |
|
|
6. Chemical |
Very reactive |
Less reactive |
|
reactivity |
Why does nitrogen show catenation properties less than carbon and phosphorus?
Phosphorus exists as discrete tetra atomic (P4).
Give preparation and properties of PCl5.
Preparation of PCl5
(i) It can be prepared by the action of excess of dry chlorine on phosphorus trichloride.
PCl3 + Cl2→PCl5
(ii) It can also be prepared by the action of sulphuryl chloride (SO2Cl2) on phosphorus or phosphorus trichloride.
Properties:
(i) When heated, it sublimes at about 430 k and dissociates
(ii) PCl5 a yellowish white powder and in moist air, it hydrolyses to POCl3 and finally gets converted to phosphoric acid.
PCl5 + H2O → POCl3 + 2HCl
POCl3 + 3H2O → H3PO4 + 3HCl
Can PCl5 act as an oxidising as well reducing agent? Justify.
PCl5 can act as an oxidizing agent not as the reducing agent.
PCl5 can act as an oxidizing agent. The highest oxidation state that P can show is +5. In PCl5, phosphorus is in its highest state (+5). However, it can decrease its oxidation state and act as an oxidizing agent .
(i) PCl5 is hydrolysed to give the oxoacid (H3,PO4) in the + 5 oxidation state.
PCl5(s) + 4H2O→ H3PO4 (aq) + 5HCl(aq)
(ii) In the gas phase, PCl5 dissociates into trihalide and the chlorine.
Justify the placement of O, S, Se, Te and Po in the same group of the periodic table in terms of electronic configuraton, oxidation state and hydride formation.
(i) Electronic configuration: They all have six electrons in the outermost shell and have ns2 np4 general electronic configuration.
(ii) Oxidation state: The outer configuration of all these elements is ns2 np4. Therefore, they complete their octet either by gaining two electrons or by sharing two electrons. Two types of oxidation states are shown by these elements.
(a) Negative oxidation state: Except the compound OF2 oxygen shows-2 oxidation state in all its compounds. Due to hgh electronegativity, it forms O2' ion in most of the metal oxides.
The electronegativities of S, Se, Te are low hence their compounds even with most electropositive elements are not more than 50% ionic. Hence S2–', Se2–' and Te2–' are less probable. Being a metal Po does not form Po2+ ion at all.
(b) Positive oxidation state: Oxygen does not show positive oxidation state except OF2(O = + 2). With the increase in atomic number of electro negativity is decreasd in this group, hence the tendency to show the positive oxidation states will increase. S, Se, Te, Po show + 4, +6 oxidation state in addition to + 2.
(iii) Hydride formation: All the elements O, S, Se, Te and Po form M2M type hydrides (where M = O, S, Se, Te and Po)
Why is dioxygen a gas but sulphur a solid?
What is the covalency of nitrogen in N2O5?

From the structure of N2O5, the covalency of nitrogen 4.
Explain why both N and Bi do not form pentahalides while phosphorus does.
What is the covalency of nitrogen in N2O5?
.

From the structure of N2O5, the covalency of nitrogen 4.
Explain why both N and Bi do not form pentahalides while phosphorus does.
thus it lack of d oribital. Nitrogen cannot increase its coordination number beyond four due to the absence of d-orbitals in its valence shell.
Bismuth does not form pentahalides due to the inert pair effect. Phosphorus forms pentahalides because it has vacant d-orbitals to extend its octet.
Account for the following observations:
among the halogens, F2, is the strongest oxidising agent?
Account for the following observations:
fluorine exhibits only-1 oxidation state whereas other halogens exhibit positive oxidation states also.
Account for the following observations:
acidity of oxo acid of chlorine is HOCl < HOCIO < HOClO2 < HOClO3
The acid strength increases:HOCl < HOCIO, < HOCIO2, < HOCIO3.
electronegativity play a role in acid strength.There are conclusions we might draw. A greater electronegativity of the atom or atoms attached to the H-O in the oxyacid apparently results in a weaker H-O bond, which is thus more readily ionized.
Since number of oxygen atom increase thus there acidic chracter is also increases.
How can you prepare Cl2 from HCI and HCI from Cl2?
(i) Cl2 from HCl: HCl can be oxidised to chlorine by the number of oxidising agent for example
By the action of any oxidising agent (whose oxidation potential is greater than Cl2) on HCl
MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
2KMnO4 + 16HCl → 2KCl + MnCl2 + 8H2O + 5Cl2
(ii) HCl from Cl2: By the direct combination of elements i.e. hydrogen and chlorine in presence of sunlight.
H2 + Cl2→2HCl
What inspired Neil Bartlett for carrying out reaction between Xe and Pt F6?
Neil Bartlett found that platinum hexa-fluoride reacts with oxygen to form a solid ionic compound of the formula [O2]+ [Pt F6]-
O2(g) + Pt F6(g)→ O2+ Pt F6–(s)
This reaction shows that Pt F6 is a very strong oxidising agent which can attract electron even from molecular oxygen. Now since the ionization potentials of oxygen molecule (1182 kj mol– 1) and Xenon atom (1170 kj mol– 1) are comparable. Bartlett believed that if PtF6 can oxidse can oxidise oxygen molecules, it should also be able to oxidise xenon atom.
What is the oxidation state of phosphorus in the following: (a) H3PO3, (b) PCl3 (c) Ca3P2, (d) Na3PO4, (e) POF3?
H3PO3
oxidation state of hydrogen is +1
oxidation state of oxygen is -2
calculation for P oxidation state is :
1 x3+ P +(-2) x3 =0
3+P+(-6)=0
3+P=6
P=6-3
=3
here oxidation state is +3
(ii) PCl3
Oxidation state of chlorine is -3
thus
P+(-3) =0
P = 3
here oxidation state is +3
(iii) Ca3P2
oxdation state of calcium is +2
2 x3 +2P =0
6+ 2P =
2P=-6
P= -6/2 =-3
oxidation state is -3
(iv)Na3PO4
here oxidation state of sodium is +1
oxidation state of oxygen is -2
1 x3 +P +(-2) x4=0
3 +P +(-8) =0
3+P =8
P=8-3 =5
oxidation state is +5
(v) POF3
oxidation state fulorine is (-1)
oxidation state is (-2)
P+(-2) +(-1) x3 =0
P= 5
oxidation state is +5
.
Write balanced equations for the following:
NaCl is heated with sulphuric acid in the presence of MnO2.
Write balanced equations for the following:
Chlorine gas is passed into a solution of NaI in water.
when chlorine gas is passed into a solution of NaI it libert iodine.
How are xenon fluorides XeF2, XeF4 and XeF6 obtained?
With what neutral molecule is CIO– isoelectronic? Is that molecule a Lewis base?
CIO– is isoelectronic with ClF. The molecule is a Lewis base.
How are XeO3 and XeOF4 prepared?
(i) Preparation of XeO3: It is prepared by the hydrolysis of XeF4 and XeF6 under controlled pH of medium.
6XeF4 + 12H2O→ 4 x e-+ 2XeO3 + 24HF + 3O2
XeF6 + 3H2O→ XeO3, + 6HF
(ii) Preparation of XeOF4
Partial hydrolysis of XeF6 gives XeOF4
XeF6 + H2O→ XeOF4 + 2HF
Arrange the following in the order of property indicated for each set:
F2, Cl2, Br2, I2 – increasing bond ethalpy.
I-I < F-F < Br-Br < Cl-Cl
Arrange the following in the order of property indicated for each set:
HF, HCl, HBr, HI - increasing acid strength
This is because as moving down the group size increase and hence weaken the bond as a result more availability of hydrogen.
H-F<H-Cl<H-Br<H-I
Arrange the following in the order of property indicated for each set:
NH3 PH3 AsH3 SbH3 BiH3 – increasing base strength.
In order of increasing base strength
NH3> PH3>AsH3>SbH3>BiH3
Give the formula and describe the structure of a noble gas species which is isostructural with
(a) ICl4– (b) IBr2– (c) BrO3–
(a) ICI4– —XeF4 (Square planar)
(b) IBr2– —XeF2(pyramidal)
(c) BrO3– — XeO3 (Linear) 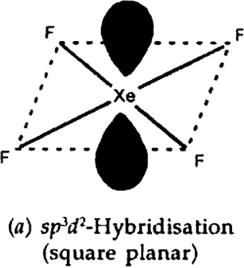

Why do noble gases have comparatively large atomic sizes?
On other hand, other element form covalent bond and it is well known that vander waal radii is larger than covalent radii. Thus noble gases have comparatively large atomic sizes.
List the uses of neon and argon gases.
neon and argon is inert gas because they are more stable than other.
Uses of Neon:
(i) Neon is mainly used in fluorescent lamps of tubes for advertising purposes. These are known as neon signs and can be seen at long distances even when there is a fog. Neon actually produces an orange red glow in the tube and on mixing with the vapours of other gases, glows or signs of different colours can be obtained.
(ii) It is used in filling sodium vapour lamps.
(iii) It is used in safety devices for protecting certain electrical instruments (voltmeters, relays, rectifiers etc.)
Uses of Argon:
(i) It is used in metal filament electric lamps since it increases the life of the tungsten filament by retarding its vapourisation.
(ii) A mixture of argon and mercury vapours is used in fluorescent tubes.
(iii) It is used to create an inert atmosphere for welding and for carrying certain chemical reactions.
State reasons for each of the following happenings:
(i) Sulphur vapour exhibits some paramagnetism.
(ii) Unlike phosphorus nitrogen shows little tendency for catenation.
(i) Sulphur vapours (S2) show some paramagnetism this is because in vapour state sulphur has two unpaired electron, like O2 due to presence of unpaired electron.
(ii) Nitrogen forms multiple bonds whereas phosphorus does not therefore, nitrogen shows little tendency for catenation. N—N bonds is weaker than P—P bond.
Give reasons for the following:
CN– ion is known but CP– is not known.
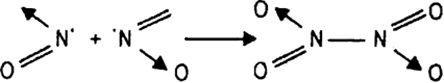
Give reasons for the following:
NO2 demerises to form N2O4
Give reasons for the following:
ICl is more reactive than I-I
The bond between two dissimilar halogen atoms is weaker than the bond between two similar halogen atoms. The overlapping between orbitals of dissimilar atom is less effective than those between similar atoms. This is because ICl is more reactive than I-I.
SF6 is a well known compond but SCl6 is not known. Explain?
Sulphur atom has a very small size. Therefore, six chlorine atoms can not be linked with an atom of sulphur whereas six fluorine atoms can be linked because of smaller size. Moreover, chlorine being less electronegative than fluorine can not cause the promotion of electrons in the vacant d-orbitals as effectively as done by fluorine because of its higher electronegativity.
Assign an appropriate reason for each of the following statements:
SiF62– is known but SiCl62– is not known.
The size of fluorine is small as compare to chlorine.
(a) The steric repulsions are less in SiF62– due to the smaller size of the F atom
(b) The interaction of the lone pairs of electrons on the F atom with Si are stronger than that of chlorine lone pairs.
Assign an appropriate reason for each of the following statements:
More metal fluorides are ionic in nature than metal chlorides.
Assign an appropriate reason for each of the following statements:
Solid phosphorus pentachloride exhibits some ionic character.
Assign reason for each of the following:
Noble gases are mostly chemically inert.
Genral electronic configuration of noble gas is ns2 np6. Thus having stable coniguration.
Noble gases are mostly chemically inert due to the following reasons:
(i) Atoms of the noble gases have stable closed shell electronic configuration.
(ii) Noble gases have exceptionally high ionisation energies.
(iii) Noble gases have very low electron affinities.
Assign reason for the following:
Bismuth is a strong oxidizing agent in a pentavalent state.
NO is paramagnetic in the gaseous state but diamagnetic in the solid and liquid states. Justify?

However, in the liquid and solid states, the unpaired electrons are involved in the formation of loose dimer. In the absence of any unpaired electrons, it is diamagnetic in nature.
(CH3)3 N is basic but (CF3)3 N is not basic. Explain.
Why the melting and boiling points of halogens increases in going from top to the bottom of the group?
Give appropriate reason for the following observation:
Only higher members of Group 18 of the periodic table are expected to form compounds.
Give appropriate reason for the following observation:
Fluorine is a stronger oxidising agent than chlorine, though fluorine has lower electron affinity than chlorine.
(ii)Fluorine has highest reduction potential due to higher hydraation energy of F– and Cl–.
Give appropriate reason for the following observation:
NO2 readily forms a dimer, whereas ClO2 does not.
Account for the following:
Chlorine water has both oxidizing and bleaching properties.
Moist chlorine gives oxygen and therefore it can act as on oxidising and bleaching agent:
Cl2 + H2O → HCI + HCIO
HCIO → HCI + O
Chlorine is an oxidising agent as it can take electrons
Cl3, + 2e– → 3Cl–
Owing to liberation of oxygen from water moist chlorine bleaches vegetable colouring matter like litmus, indigo, lien wood pulp, cotton fabric etc.
Cl2, + H2O → 2HCl + [O]
Coloured matter + [O] → Colourless matter
Account for the following:
H3PO2 and H3PO3 acts as good reducing agents while H3PO4 does not.
in H3PO2 and H3PO3 , 2 hydrogen atoms and 1 hydrogen atom respectively are bonded to phoshorous atom. But in the case of H3PO4 , there is no hydrogen atom bonded to phosphorous atom. So, the oxidation state is lower in H3PO2 in comperision to H3PO4 so it is not a reducing agent.
Account for the following:
On addition of ozone gas to KI solution, violet vapours are obtained.
On addition of ozone gas to KI solution. Ozone is oxidizing iodide ion to I2 the elemental from of iodine thus elemental iodine readlily undergoes sublimation to form the violet gas.
2KI + H2O+ O3 → 2KOH + I2 + O2
SF6 is known but SCl6 is not known.
NF3 does not have donor properties like ammonia. Explain.
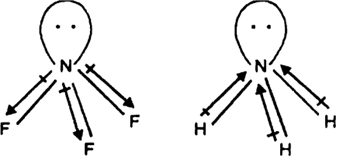
The lone-pair on N is in opposite direction to the N—F bond moments and therefore it has very low dipole moment (about 0.234 D). Thus it does not show donor properties. But ammonia has high dipole moment because its lone pair is in the same direction as the N—H bond moments. Thus it has donor properties.
The electronegtivity also influence the donar properties. fluorine is more electronegtive than hydrogen thus fluroine pull electron from nitrogen but hydrogen is not.
PCl5 exists as [PCl6]– [PCl4]+ but PBr5 exists as [PBr4] + [Br]–. Explain.
On the other hand, PBr5 splits up into stable tetrahedral structure as [PBr4]+ [Br]–
This splitting is different from PCl5 because Br atoms are large and six atoms of Br cannot be easily accommodated around smaller P atom.
SOCl2 can act as a weak Lewis acid as well as a weak Lewis base. Explain.
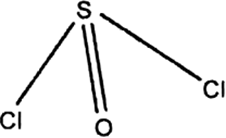
It has a pyramidal structure involving sp3 hybridisation with a lone-pair of electrons as:
So, Lewis basic character is due to the presence of a lone-pair. In addition SOCl2 has also empty d-orbitals which can be used to accept electron pairs and hence it behaves as a Lewis acid.
Describe the chief uses of fluorine, chlorine and their compounds.
Uses of fluorine:
(i) It is used in the preparation of fluoro carbon which are non-inflammable and chemically inert and are used as solvents, lubricants and insulator.
(ii) It is used as a refrigerant in many of the cooling processes.
(iii) It finds considerable use as DDFT, which similar to DDT, is extremely; efficient as a fungicide and fumigant.
(iv) In nuclear physics and higher voltage electricity, fluorine as SF6 finds very great use e.g., in the separation of isotopes of uranium.
Uses of chlorine:
(i) It is used in sterilization of drinking water.
(ii) Large quantities of chlorine are used for bleaching paper pulp and textiles.
(iii) It is used in the manufacture of inorganic chemicals such HCl, sodium hypochlorite (NaOCl), bleaching powder (CaOCl2), phosphorus trichloride (PCl3) phosphorus pentachloride (PCl5) etc.
(iv) It is used in the manufacture of vinyl chloride which is the starting material for the plastic polyvinyl chloride (PVC).
(v) It is used in the manufacture of insecticides like DDT, germicides, dyes and drugs.
Knowing the electron gain enthalpy values for O O– and O O2– as -141 and 702 kJ mol–1 respectively, how can you account for the formation of a large number of oxides having O2-species and not O–?
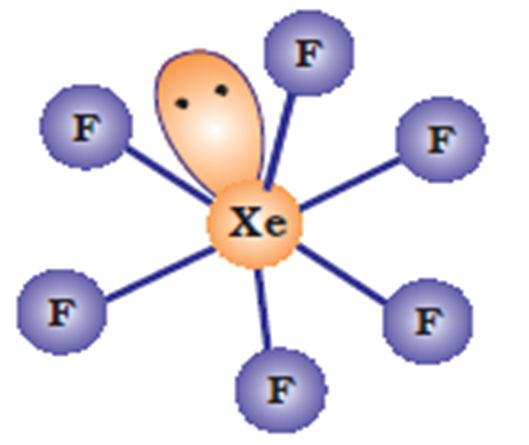
Using VSEPR theory, predict the structures of SO3,2– IF7–, XeF2, ClO4–, ICl4– and IBr2–.
(ii) Structure of IF7: Total number of electrons in the valency shell of the central atom, i.e., I = 7.
No. of electrons provided by the seven F atoms = 7 x 1 = 7.
Total no. of electrons around the cental atoms i.e., = 7 + 7 = 14.
Therefore, total no. of electron pair around the central atom i.e., = 14/2 = 7.
But the total no. of bond pairs = 7.
(Because there are seven I—F bonds)
Therefore, total no. of lone pairs = 7 – 7 = 0 on the basis of VSEPR theory, a molecule with seven bond pairs and no of lone pair must have pentagonal bipyramidal geometry.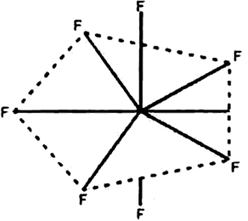
Fig. Shapes of IF7 molecule.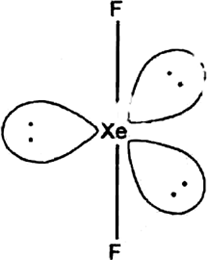
Fig. Structure of xenon difluoride.
Structure of XeF2: Xenon difluoride molecule possesses a trigonal bipyramidal structure. The xenon and fluorine atoms lie in a straight line (linear position) while the three lone pairs of xenon occupy the equatorial positions.
Structure of ClO–4: It has tetrahedral shape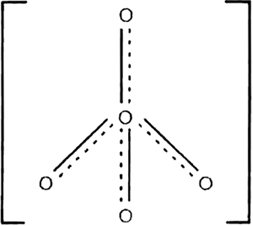
Fig. Shapes of ClO4–.
Structure ICl4–
centre iodine contain 8 electron (7 from iodine and one form negtive charge) In which 4 bond pair and 2 lone pair total 6 pair of electron
thus strycture of ICl4– is square planer 
centre iodine contain 8 electron (7 from iodine and one form negtive charge) In which 2 bond pair and 3 lone pair total 5 pair of electron
thus strycture of IBr2– is linear structure IBr2–.
Assign a reason for each of the following statement:
Ammonia is a stronger base than phosphine.
Answer the following:
Of Bi(V) and Sb(V) which may be a stronger oxidising agent and why?
and configuration of Bi is 4f145d10 6s2 6p3.
Onmoving down he group the stability of +5 oxidation state decrease while that of +3 oxidation state increase due to inert pair effect (due to presence of f orbital). Thus +5 oxidation state of Bi is less stable than +5 oxidation of Sb.
Therefore Bi(V) is more stronger oxidising agent thanSb(V).
Assign a reason for the following statement?
Phosphorus shows marked tendency for catenation but nitrogen shows little tendency for catenation.
The lesser tendency of nitrogen to show catenation in comparison to phosphorus is their law (M–M) bond dissociation energy.
Bond N–N P–P
Bond energy (KJ mol-1) 163.8 201.6
Assign a reason for the following statement?
The electron gain enthalpy with negative sign for oxygen (–141'k] mol–1) is less that for sulphur (–200 kj mol– 1).
Write chemical equation for the following process:
Orthophosphorus acid is heated.
Write chemical equation for the following process:
PtF6 and xenon are mixed.
Complete the following chemical equation:
Complete the following chemical equation:
Give reasons for the following:
(a) PCl5 acts as a chlorinating agent in organic reactions.
(b) Nitric oxide becomes brown when released in air.
(c) PCl5 is ionic in nature in the solid state.
(d) Ammonia acts as a ligand.
(e) Sulphur disappears when boiled with an aqueous solution of sodium sulphite.
(b) It is because N6 reacts with O2 to form nitrogen dioxide which is brown in colour
(c) PCl5 is ionic in nature in solid state because it exists as [PCl4]+ [PCl5]–
(d) NH3 has lone pair of electron, therefore, acts as ligand.
(e) It is due to formation of sodium thiosulphate.
A translucent waxy solid (A) on heating in an inert atmosphere is converted to its allotropic from (B). Allotrope (A) on reaction with very dilute aqueous KOH liberates highly poisonous gas (C) having rotten fish smell. With excess of chlorine forms (D) which hydrolyses to compound (E).
Identify compound (A) to (E)
Or
Concentrated sulphuric acid is added followed by heating to each of the following test tube labelled (i) to (v).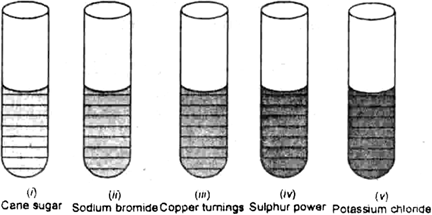
Identify in which of the above test tube the following change will be observed. Support your answer with the help of a chemical equation.
(a) Formation of black substance
(b) Evolution of brown gas
(c) Evolution of colourless gas
(d) Formation of brown substance which on dilution becomes blue.
(e) Disapperance of yellow powder along with evolution of colourless gas.
A is white phosphorus.
B is red phosphorus.
C is phosphine
D is phosphorus trichloride
E is phosphorus acid.
OR
(a) In test (i), there will be observed.
(b) In test (iii), there will be evolution of brown gas
(c) In test tube (v), there will be evolution of colourless gas
(d) In test tube (d), there will be formation of brown substance which on dilution becomes blue.
(e) In test tube (iv), there will be disappearance of yellow powder along with evolution of colourless gas.
Fluorine has lower electron affinity than chlorine and yet it is a stronger oxidising agent than chlorine. Explain.
i)when element combine with fluorine the energy released are highest due to small in size of F.
ii)lattice energy and hydration energy of fluoride are higher those of chlorides.
iii) F2 has a low dissociation energy than Cl2 because of the repulsion between the lone pair on the two F and the non existance of multiple bonding involving d - orbitals.
Of HI and HCl which has a weaker covalent bond and what effect has it on their acid strengths?
NaOCl solution becomes unstable on wanning. What happens to it?
it disproportionates to NaCl and NaClO3.
Draw the structures of XeF4 and SF4 molecules.

Structure of SF4 trigonal pyramidal

Discuss the anamolous behaviour of fluorine among the halogens. Give reason also.
(A) Fluorine shows abnormal behaviour because of the following facts:
(i) Smaller atomic size.
(ii) Higher electronegativity.
(iii) Non-availability of empty d-orbitals in its valency shell.
The main points of difference are:
(i) Fluorine exhibits on oxidation state-1 only whereas remaining halogens may exhibit oxidation states –1, + 1, + 3, – 5 and + 7. The higher oxidation states arise due to the presence of vacant d-orbitals in their valency shells.
(ii) On account of high. electronegativity fluorine enters into hydrogen bond formation in its compounds with hydrogen. Thus hydrogen fluoride is an associated molecule due to hydrogen bonding.
![]()
The phenomenon of hydrogen bonding is not shown by other hydracids.
(iii) Reactivity: Fluorine is most reactive among the halogens. This is due to high electronegativity, small size of its atom, extremely high oxidizing power and its low F—F bond energy (38.5 k cal/ mole). This is indicated by the following properties
(a) Combination with hydrogen: Fluorine reacts with hydrogen in dark at a low temperature. The other halogens do not react with hydrogen in dark.
(b) Action with metals : Fluorine reacts with metals like gold and platinum. The other halogens do not react with these metals.
(c) Action with non-metals: Fluorine combines directly with the non-metals like carbon, silicon, nitrogen etc. to give their fluorides.
The other halogens do not combine directly with these elements.
(iv) Action with water : Fluorine reacts with water forming HF, O2, and O3.
The other halogens do not give ozone with water.
(v) Action with alkalies: Fluorine reacts with caustic alkalies to form oxygen difluoride
The other halogens react with cold and dilute alkalies to form hypohalites and with hot and conc. alkalies to form higher oxy salts, halates, with cold and dilute alkali:
with hot and conc. alkali
vi) Formation of oxy acids: Fluorine does not form any oxyacid because it is the strongest oxidising agent while the remaining halogens form four types of oxyacids.
HXO — Hypohalous acid
HXO2 — halous acid
HXO3 — Halic acid
HXO4, — Per-halic acid.
(vii) Behaviour of hydracids:
(a) HF is a liquid whereas other hydracids HCI, HBr HI are gases at ordinary temperature.
(b) HF is a weak acid, while the other hydr– acids are strong acids.
(c) HF is most stable of all the hydracids of remaining halogens.
(d) HF forms acid salts such as NaHF2 while the other halogens acids do not form such salts.
(e) HF can form complex acids such as HBF4, H2SiF6, while the other halogen acids do not form such acids.
(f) HF is the only acid which reacts with silica, silicates and hence attacks glass.
(viii) Behaviour of salts:
(a) AgF is soluble in water, whereas other silver halides (AgCl, AgBr, Agl) are insoluble.
(b) The fluorides of calcium, strontium and barium are insoluble in water, whereas corresponding salts of other halogens are soluble.
(B) (i) Electron affinity: Cl > F > Br > I.
(ii) Oxidizing power: F2 > Cl2, > Br2 > I2.
Discuss the general characteristics of Group 15 elements with reference to their electronic configuration, oxidation state, atomic size, ionization enthalpy and electronegativity.
(i) Electronic configuration: The valence shell electronic configuration of these elements is ns2 np3. The s orbital in these elements is completely filled and p orbitals are half filled, making their electronic configuration extra stable.
(ii) Atomic Size: Covalent and ionic (in a particular state) radii increase in size down the group. There is a considerable increase in covalent radius from N to P. However, from As to Bi only a small increase in covalent radius is observed. This is due to the presence of completely filled d and f or f orbitals in heavier members.
(iii)Oxidation State: The common oxidation states of these elements are –3, + 3 and + 5. The tendency to exhibit –3 oxidation state decreases down the group due to increase in size and metallic group. In the last member of the group, bismuth hardly forms any compound in –3 oxidation state. The stability of + 5 oxidation state decreases down the group. The stability of + 5 oxidation state decreases and that of + 3 state increases (due to invert pair effect) down the group. Nitrogen exhibits + 1, + 2, + 4 oxidation states also when it reacts with oxygen. Phosphorus also shows +1 and + 4 oxidation states in some oxo acids.
(iv) Ionization enthalpy: Ionization enthalpy decreases down the group due to gradual increase in atomic size. Because of the extra stable half filled p orbitals electronic configuration and smaller size, the ionization enthalpy of the group 15 elements is much greater than that of group 14 elements in the corresponding periods. The order of successive ionization emthalpies are expected as ΔH1, < ΔH2 < ΔH3.
(v) Electronegativity: The electronegativity value, in general, decreases down the group with increasing atomic size. However, amongst the heavier elements, the different is not that much pronounced.
Assign reasons for the following:
Ammonia (NH3) has greater affinity for protons than phosphine (PH3).
Assign reasons for the following:
The negative value of electron gain enthalpy of fluorine is less than that of chlorine.
Assign reasons for the following:
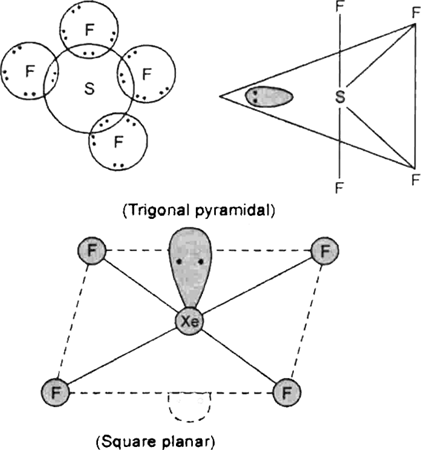
SF6, is much less reactive than SF4.
In SF6 all of the electron are paired giving a great stability to the molecule and reducing its reactivity.
Assign reasons for the following:
Of the noble gases only xenon is known to form well-established chemical compounds.
Discuss the favourable conditions for the manufacture of (i) ammonia by Haeber's process and (ii) sulphuric acid by contact process.
(i) Favourable conditions for the manufacture of ammonia by Haeber Process:
(i) A high temperature of 400-500°C.
(ii) A high pressure of 200-1000 atoms.
(iii) A catalyst usually Fe + Mo or finely divided iron and Fe3O4 containing small amounts of K2O and Al2O3.
(ii) Favourable conditions for the manufacture of sulphuric acid by contact process:
(i) High concentration of oxygen : Air or oxygen used for the oxidation of sulphur dioxide to sulphur trioxide must be in excess. Furthermore, these gases must be absolutely pure otherwise they will poison the catalyst.
(ii) High pressure: Since the forward reaction proceeds with decrease in volume, therefore, high pressure will favour the reaction. In actual practice, a pressure of about 2 atmospheres is used. This is because gases are acidic and corrosion of the plant occurs at high pressure.
(iii) Low temperature : Since the forward reaction is exothermic, therefore, low temperature will favour the reaction. However, rate of the reaction decreases with decrease in temperature. Therefore, the reaction is carried out at an optimum temperature of 623-723 K.
(iv) Use of catalyst: To increase the rate of a reaction at low temperature, a catalyst is to be used. The commonly used catalysts are platinum, or divanadium pentoxide (V2Os). Since platinum is quite costly and is easily poisoned by arsenic impurities usually present in SO2, therefore, these days, divanadium pentoxide is employed because it is not only cheaper but is also not easily poisoned.
(v) Purity of gases: Purity of gases is another essential conditions for the maximum yield of SO2. The impurities present in the reacting gases act as catalytic poison and thus decrease the efficiency of the catalyst.
Draw the structures of the following:
(i) PCl5(g) (ii) S8(g) (iii) ClF3(g).
(ii) Structure of S8:
(iii) Structure of CIF3:

Complete the following chemical reaction equation:
F2 + H2O → ________
Draw structures of the following species:
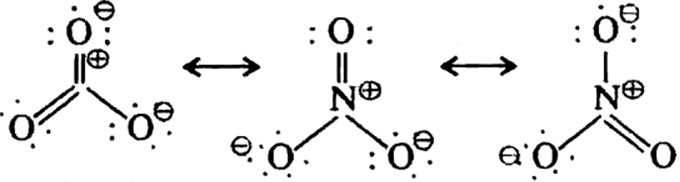
(i) H2,S2,O7 (ii)NO3–.
(ii) Structure of NO3–: The structure of the nitrate ion is a triangular planar. All three oxygen atoms are equivalent in a resonance hybrid.
Assign a reason for the following statement:
Phosphorus (P4) is more reactive than nitrogen (N2).
Assign a reason for the following statement:
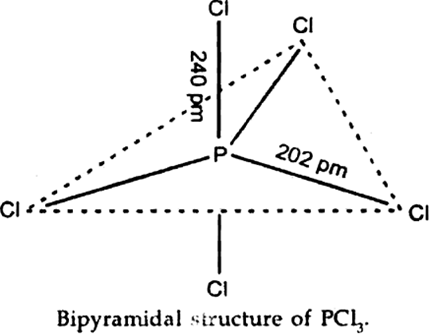
All the bonds in PCl5 are not equal in length.
The three equatorial P–Cl bonds are equivalent, while the two axial bonds are longer than equatorial bonds. This is due to the fact that the axial bond pairs suffer more repulsion as compared to equatorial bond pairs.
Write the structural formulae of the following compounds:
(i) BrF3 (ii) XeF2.
Structural formula of :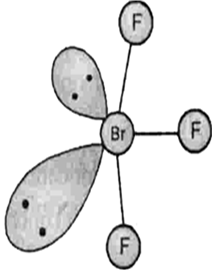
Structural formula of 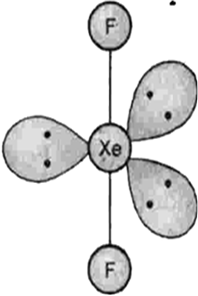
Chlorine is liberated from hydrochloric acid in cold by the action of __________.
Hydrogen Sulphide reduces hot concentrated sulphuric acid to _________ and hot concentrated nitri acid to _________.
Sulphur dioxide
,nitrogen dioxide
State whether the following statements are true (T) or false (F):
A.
Argon is the most abundant noble gas in the atmosphere.B.
Sulphur, phosphorus and oxygen are the element which shows allotropy.C.
XeF4 molecule has a tetrahedral shape.D.
Among halogens, F2 has a lowest bond dissociation enthalpy.E.
Among all the halogens, only iodine forms polyhalide ion.B. TRUE
C. FALSE
D. TRUE
E. TRUE
State whether the following statements are true (T) or false (F):
A.
Nitrogen does not extend its covalency beyond four.B.
The group 16 elements have general electronic configuration ns2 np3.C.
H2SO4 is a dehydrating and oxidising agent.D.
Pure phosphine is inflammable.E.
Nitrous oxide does not support the burning splint.B. FALSE
C. TRUE
D. FALSE
E. FALSE
State whether the following statements are true (T) or false (F):
A.
Chlorine gas undergoes disproportionation when passed into water.B.
The reaction betwen hydrogen and fluorine is very low at room temperature.C.
HBr is a stronger acid than HI because of hydrogen bonding.D.
Iodine is a better nucleophile than bromide.E.
Among halogens, iodine is the most reactive.B. FALSE
C. FALSE
D. TRUE
E. TRUE
PCi5 exists but NCi5 does not because
- N2 is inert
- NCl5 is unstable
- small size of N
- of non-availability of vacant d-atomic orbitals.
D.
of non-availability of vacant d-atomic orbitals.The shape of IF7 molecule is
- tetrahedral
- trigonal bipyramidal
- octahedral
- pentagon bipyramidal
D.
pentagon bipyramidalThe most stable allotropic of sulphur is
rhombic sulphur
- flowers of sulphur
- plastic sulphur
- monp-clinic sulphur
A.
rhombic sulphur
Iodine can exist in the oxidation states - + 1, + 3, + 5
- –1, + 1, + 3
- + 3, + 5, + 7
- –1, + 1, + 3, + 5, + 7
D.
–1, + 1, + 3, + 5, + 7 Catalytic oxidation of NH3 gives - dinitrogen pentoxide
- nitric oxide
- nitrogen dioxide
- nitrogen
B.
nitric oxideOxygen molecule exhibits
- Paramagnetism
- Dimagnetism
- Ferromagnetism
- Ferrimagnetism
A.
ParamagnetismOxygen molecule exhibits
- Paramagnetism
- Dimagnetism
- Ferromagnetism
- Ferrimagnetism
A.
ParamagnetismWhen SO2 is passed through acidified K2Cr2O7 solution
- The solution turns blue
- The solution is decolourised
- SO2 is reduced
- Green Cr2(SO4)3 is formed
D.
Green Cr2(SO4)3 is formedWhat type of hybridisation is associated with N in NH3? What is the expected bond angle in NH3?
NH3 is Sp3 hybirdisation as there are three bonding pairs of electrons and one non bonding pair. The bond angle in a molecule of ammonia are 107o, a value very close to tetrahedral angle (109o.5').
The central atom has both shared and unshared electron pairs. The shape of ammonia molecule (NH3) is trigonal pyramid.

why is bond dissociation of energy of fluorine molecule less than that of chlorine molecule ?
It is because of the small atomic radius of Fluorine. Fluorine has quite a small atomic radius compared to the Chlorine. Fluorine gas is diatomic. Since the atomic radius of Fluorine is very small, the nuclei of both the Fluorine atoms repel each other(like charges repel), Fluorine gas(F2) has a really small dissociation energy compared to the chlorine
What are the two important oxidation states of group 16 elements in the periodic table?
Group 16 elements show oxidation property, the stability of -2 oxidation state decreases down the group.
Polonium hardly shows –2 oxidation state. Since electronegativity of oxygen is very high, it shows only negative oxidation state as –2 except in the case of OF2 where its oxidation state is + 2. Other elements of the group exhibit + 2, + 4, + 6 oxidation states but + 4 and + 6 are more common. Sulphur, selenium and tellurium usually show + 4 oxidation state in their compounds with oxygen and + 6 with fluorine.
What type of hybridisation explains the trigonal bipyramidal shape of SF4?
SF4 have Sp3d hybridisation. SF4 is not square planar. SF4 has a seesaw geometry. Sulfur tetrafluoride has 10 electrons around the central sulfur atom. This means there are five electron pairs are present. The molecule has four S-F bonds and just one lone pair. Lone pair will occupy the place with least LP-bond repulsion. If LP occupies axial position in trigonal bipyramid, it will 'feel' three S-F bonds nearby. If LP occupies equatorial position, it will 'feel' only two S-F bonds, as other two will be pushed more away.
What is the state of hybridization of N in NO3-?
The sp3 hybrid orbitals of the central atom N is displayed. The three sp2 orbital lie in a plane and form a trigonal planar arrangement. Each of the N-O bond is formed by the overlap of a nitrogen sp2 hydride orbital and an oxygen 2p orbital. The NO3- molecule is planar and all ONO angles are 120°.
Name the geometry of XeF4 and XeO3.
XeF4: Octahedral hybridization: Sp3d2
The geometry of XeF4 is a square planar with symmetric electron reigon distribution.
XeO3 : Tetrahedral Hybridization : Sp3
The molecular geometry of XeO3 is trigonal pyramidal with asymmetric charge distribution on a central atom.
Why is N2 not particularly reactive?
Nitrogen is inert molecule because in nitrogen there exist a triple bond betwwn the two nitrogen atoms.
This triple bond constituted of one sigma and two pi bonds i.e.
Px -Px overlap to form sigma bond.
Py-Py and Pz-Pz overlaps to form pi bond.
The intermolecular forces in this are weak Van der Walls forces. So, the diatomic molecules exist freely.Because of the triple bond,the bond energy is very high.The bond dissociation energy of nitrogen is 945.4 kJ/mol.Due to this high bond dissociation energy, nitrogen is apparently inactive under normal conditions.This is why nitrogen is unreactive gas.
Give an example of oxoacid of phosphorus in which oxidation state of phosphorus is + 4.
Hypophosphoric acid is oxoacid of phosphorus in which oxidation state of phosphorous is +4.
Formula of hypophosphoric acid is H4P2O6
Calculation of oxidation state.
hydrogen have +1 oxidation state and oxygen has -2 oxidation state thus
1 x4 + 2P +(-2) x 6 = 0
4+2P-12=0
4+2P=12
2P=12-4
2P=8
P=8/2
P=4
Name the noble gas which has (i) highest first ionization energy (ii) highest boiling point.
i) In noble gas helium has the highest first ionization energy.
This is beause smaller the size greater will be the force of attraction and thus required more energy to remove an electron from a helium atom.
As we move down the group the first ionization energy become smaller. The force of attraction of the positively charged nucleus for electrons decrease as the square of the distance between them increase.
ii) Xenon has highest boiling point. In noble gases only london dispersion force take place between them.
These dispersion forces are momentary i.e, they develop only for a fraction of time and the strength of these forces depend on the distortion of electron cloud of the atom by another atom.
Now, greater the size of the atom greater will be the distoration of electron cloud of the atom and stronger will be the dispersion force which will in turn increase boiling point.
Why does sulphur in vapour state exhibit paramagnetic character?
Sulphur exist as S8 molecule at ordinary temperature and pressure but at elevated temperature it gets dissociated and partly exists as S2 molecule in vapor phase.
In vapor phase S2 has two unpaired electron in its antibonding pi molecular orbitals and behave as paramagnetic material.
Arrange the hydrides of group 16 in the decreasing order of their thermal stability. Is the order same or different for their reducing character?
a) The thermal stability of hydrides decrease on moving down the group this is because of decrease in the bond dissociation enthalpy of hydrides.
H2O>H2S>H2Se>H2Te>H2Po
b) Reducing character:
All hydrides of group 16 elements, except H2O, are reducing agent.
The reducing power of these hydrides increase in going from H2S to H2Te, which may be due to increase in the size of the atom and hence decrease in the M-H bond energy.
Arrange the hydrides of group 16 in the decreasing order of their thermal stability. Is the order same or different for their reducing character?
The stability of hydrides decreases moving down the group this is because of the decrease in the bond dissociation enthalpy of hydrides on moving down the group.
H2O>H2S>H2Se>H2Te>H2Po
Reducing Character:
All hydrides of group 16 elements, except H2O, are reducing agent.
The reducing power of these hydrides increase in going form H2S to H2Te, which may be due to increase in the size of the atom and hence decrease in the M-H bond energy.
How is sulphur tetrafluoride prepared?
SF4 is produced by the reaction of SCl2, Cl2 and NaF.
SCl2 +Cl2 +4NaF → SF4 +4NaCl
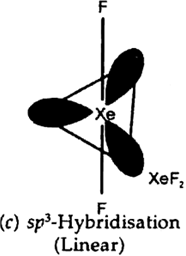
Why is XeF2 linear in shape?
There are five electron pair around the xenon (two is bonding pairs and three lone pair)The arrangement of molecule is Trigonal bipyramidal. The shape is linear beacuse lone pair prefer equatorial position.
Why does fluorine not form oxoacids?
flourine is very electronegative and it cannot exist in positive oxidation states in its compounds .in oxyacids of halogens ,oxygen carries a negative charge while the halogen carries a positive charge.the electronegativity of flourine is 4,while that of oxygen is 3.5. so due to high value of electronegativity ,flourine shows reluctance to form positive oxidation states and donot form oxyacids.
While other halogen form a number of oxoacid i which they have +1,+3,+4, and +6 oxidation state.
F forms only one oxoacid HOF which is unstable.
Assign reason for the following:
In solid state PCl5 behaves as an ionic species.
Give reason for the following:
Among the noble gases only xenon is well known to form chemical compounds.
Only xenon is well known to form chemical compounds, because xenon is large in size and having higer atomic mass.
Due to having larger atomic radius the force of attraction between the outer electron and the protons in the nucleus is weaker.
Hence they easily available to form compound.
Write chemical reaction to show that chlorine gas can be obtained from bleaching powder.
When bleaching powder react with water, it forms calcium hydroxide and chlorine gas.
CaOCl2 +H2O ---> Ca(OH)2 +Cl2
Give chemical evidence for the following:
Fluorine is a stronger oxidising agent than chlorine.
Fluorine is a stronger oxidising agent than chlorine because of having high electrode potential and also having a strong tendency to accept electron and hence they are strong oxidising agent.
Draw the structure of P4O10 and identify the number of single and double P — O bonds.
In this structure there are four P=O bond.
Structure of P4O10 
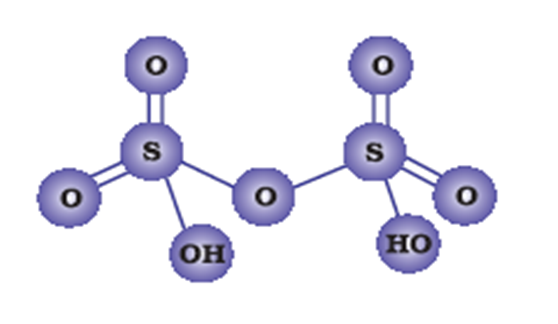
Draw molecular structures of (i) peroxo disulphuric acid, (ii) Iodine pentafluoride.
structure of peroxo disulphuric acid.
Structure of Iodine pentafluoride.
How is XeO3, prepared? Write the related chemical equations. Draw the structure of XeO3.
Xenon trioxide is an unstable compound of xenon, it has +6 oxidation state.
Xenon trioxide may be prepared by the hydrolysis of XeF4 or XeF6 .
XeF6 +3H2O ----->XeO3 +6HF
6XeF4 +12H2O----->2XeO3 +4Xe +3O2 +24HF
Explain why phosphorus forms PF5 while nitrogen does not form NF5.
Nitrogen is small in size and it cannot stabilize five flourine but phosphorus can stabilize five fluorine atom because of larger in size and it also have vacant d- orbitals. So due to having extra space phosphorus form PF5 .
Accounts for the following:
NH3 is stronger base than PH3.
Nitrogen is the smallest member in group. Hence the lone pair of electron on nitrogen can be easily donated. On the other hand Phosphorus is bigger size than nitrogen and therefore the, lone pair of electron is not readily available for donation. More readily a species donate an electron pair, more basic it is. Thus NH3 is stronger base than PH3
Accounts for the following:
HF is weaker acid than HI
The electronegativity difference between hydrogen and fluorine is very large which means that these two element will have a very strong attraction to each other.
Stronger the force of attraction lesser have capcity to donate.
Are all the five bonds in PCl5,molecule equivalent? Justify your answer.
PCl5 has a trigonal bipyramidal structure and the three equatorial P-Cl bonds are equivalent while the two axial bonds are different and longer than equatorial bonds.
This is because having greater bond pair -bond pair replusion.
Explain why ClF3 exists whereas FCl3 does not.
Chlorine has empty d-orbital and it acquires excited state at the time of bonding when electron from 3p-orbital are promoted to 3d- orbital.
In first excited state chlorine atom can exhibit a covalency of three, hence cannot expand its octetdur to absence of empty d- orbitals in 2nd energy shell.
Hence, it cannot exhibit covalency more than 1therefore FCl3 is not possible.
How would your prepare the following: (i) H3PO3, (ii) HI, (iii) HClO4.
i)Phosphorous acid can be form as
PCl3 +3H2O (cold) →H3PO3
(ii)HI formed by the reaction of I2 with hydrazine which also yield nitogen gas.
2I2+N2H4→ 4HI +N2
iii) sodium hypochlorite react with hydrochloric acid to give perchloric acid.
NaClO4 +HCl →NaCl +HClO
How are XeF2, and XeF4 prepared?
XeF4 and XeF2 can be prepared by the direct reaction of xenon and fluorine.
Xe(g) +F2(g) ----> XeF2(s)
Xe(g) +2F2(g) ----> XeF4(s)
Draw the structures of the followings: (a) H2SO3, (b) H2SO4, (c) SO3.
(i) Structure of H2SO3
ii) structure of H2SO4
iii) structure of SO3
Account for the following:
The boiling points of noble gases increase with the increase in atomic number.
The melting and boiling points of noble gases are very low in comparison to those of other substances of comparable atomic and molecular masses. This indicates that only weak van der Waals forces or weak London dispersion forces are present between the atoms of the noble gases in the liquid or the solid state.
The van der Waals force increases with the increase in the size of the atom, and therefore, in general, the boiling point increase from He to Rn.
Account for the following:
Neon is generally used in warming signal illumination.
Neon gas is used in warning signals because when electric current is applied to it, it emits an orange/red colour light. As we know orange/red light is used for warning signals it makes it very useful to use the tubes containing neon gas for producing warning signals.
Account for the following:
For protecting electrical instruments, neon is generally used in safety devices.
Neon is used in electrical instrument for the protection because of its characteristic property of caarrying exceedingly high curent under high voltage, it is used in safety devices for protecting electrical instrument such as voltameter from high voltage.
How is oxygen converted into ozone?
a) Ozone is an allotropic form of oxygen. It is too reactive to remain for long in the atmosphere at sea level. At a height of about 20 kilometres, it is formed from atmospheric oxygen in the presence of sunlight. This ozone layer protects the earth’s surface from an excessive concentration of ultraviolet (UV) radiations.
I) In the fist step oxygen molecule break down to give oxygen radical.
O2 +hv→ O* +O*
II) These oxygen radical react with oxygen molecule to form ozone.
O2 +O* → O3
When a slow dry stream of oxygen is passed through a silent electrical discharge, conversion of oxygen to ozone (10%) occurs. The product is
known as ozonised oxygen.
3O2 → 2O3 (298 K) = +142 kJ mol–1
Since the formation of ozone from oxygen is an endothermic process, it is necessary to use a silent electrical discharge in its preparation to
prevent its decomposition.
Write chemical equations for the action of ozone on silver and on silver oxide.
Silver metal when warmed with ozone gets blackened due to reduction of the oxide formed in the initial stages of the reaction.
2Ag +O3 → Ag2O +O2
Ag2O +O3→ 2Ag +2O2
Discuss the anomalous behaviour of nitrogen in group 15 elements. What is the cause of this anomalous behaviour?
Anomalous behaviour of nitrogen:
I)Nitrogen differ from of the members of this group due to its small size, high electronegativity, high ionisation enthalpy and non-availability of d orbitals.
II)Nitrogen has unique ability to form
pπ -pπ multiple bonds with itself and with other elements having small size and high electronegativity (e.g., C, O).
III)Heavier elements of this group do not form pπ -pπ bonds as their atomic orbitals are so large and diffuse that they cannot have effective overlapping.
IV) nitrogen exists as a diatomic molecule with a triple bond (one s and two p) between the two atoms. Consequently, its bond enthalpy
(941.4 kJ mol–1) is very high. On the contrary, phosphorus, arsenic and antimony form single bonds as P–P, As–As and Sb–Sb while
bismuth forms metallic bonds in elemental state. However, the single N–N bond is weaker than the single P–P bond because of high
interelectronic repulsion of the non-bonding electrons, owing to the small bond length. As a result the catenation tendency is weaker in nitrogen.
(Oxides of nitrogen have open chain structures while those of phosphorus have closed chain or cage structures). Why is it so? Illustrate with one structural example for each type of oxides.
Nitrogen is small in size and have ability to form multiple bonding with oxygen.thus oxides of nitrogen have open chain structures. For example N2O5.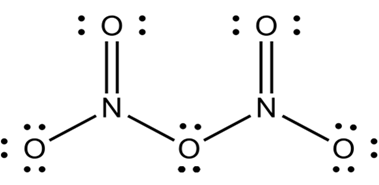
On other hand phosphorus due to its larger size does not form this type of multiple bonds with oxygen but instead forms single bonds and forms oxides with cage like structure.
For example P4O10.
Why are inter-halogen compounds more reactive than the corresponding elemental hydrogen?
Inter-halogen compounds more reactive than the corresponding elemental hydrogen because interhalogen are weaker than diatomic halogen bond except for F2.
It is due to their differences in electronegtivies. A substance like ICl is more reactive than Ioinde(I2) because the electronegtive difference polarise the bond between Iodine and chlorine and therefore breaks more easily.
Write the structures of following species:
(i) H3PO2 (ii) H2SO4
I) Structure of H3PO2
II) Structure of H2SO4
How would you account for the following:
Hydrogen fluorine is much less volatile than hydrogen chloride.
How would you account for the following:
Interhalogen compounds are strong oxidising agents.
Interhalogen compound are strong oxidising agent, it due to their difference in electronegtives which make bond more polar and therefore easily breaks.
hence act as oxidising agent.
How would you account for the following:
Sulphur hexafluoride is less reactive than sulphur tetrafluoride.
Sulphur hexafluoride is less reactive than sulphur tetrafluoride because SF6 is sterically hindered by the six fluorine atom.
On other hand SF4 have one lone pair, Hence tetrafluoride is more reactive.
How would you account for the following:
In the noble gases only xenon forms known chemical compounds.
Only a small amount of energy is needed to excite the pair of electron from the 5p orbital to the 6s and 4f electrons where they can then form covalent bond with other atom.
for example XeF6
Compare the structural shapes of the following species: SF6 and SF4.
The shape of SF4 is see saw geometry.
SF6 has an octahedral geometry.

Write balanced chemical equations for the following reactions:
(a)
(b)
a) Ca3P2 +H2O→ 3Ca(OH)2 +2PH3
b) XeF6 + 3H2O → XeO3 + 6HF
Explain the following:
(i) Most of the known noble gas compounds are those of Xenon.
(ii) ClF3 exists but FCl3 does not.
(iii) Among the hydrides of elements of Group 16, water shows unusual physical
properties.
(iv) Unlike phosphorus, nitrogen shows little tendency for catenation.
(v) Despite lower electron affinity, fluorine is a stronger oxidising agent than chlorine.
Only a small amount of energy is needed to excite the pair of electron form the 5p orbital to the 65 and 4f electrons where they can then form covalent bonds with other atom.
ii) The electronic configuration of chlorine atom 1s2 2s2 3s2 3p6 3d0 it has vacant d orbitals it, therefore, can expand it valence shell and show +3 oxidation state, Hence ClF3 is formed.
On other hand, flourine atom 1s2 2s2 2p5 has no d - orbital and, therefore, it can not expand its valence shell. It only shows -1 oxidation state. Hence FCl3 does not exist.
iii) The oxygen has high electronegativity. Hence the O-H bond in H2O forms strong intermolecular hydrogen bonds. As a result, water exist as an associated molecule. But the other hydrides of group 16 do not form hydrogen bonds. Therefore they exist as discrete molecules. Thus, water shows unusual physical properties such as high thermal stability, high b.p., weaker acidic character as compared to the other hydrides of group 16.
iv) Nitrogen is small in size and have unique ability to form multiple bonding; single N–N bond is weaker than the single P–P bond because of high interelectronic repulsion of the non-bonding electrons, owing to the small bond length. As a result the catenation tendency is weaker in nitrogen.
v) Halogens have high electronegativity and electron affinity. They have greater tendency to accept electrons or easily reduced, therefore they are strong oxidizing agent.
Discuss the properties of oxygen, sulphur, selenium, tellurium and polonium (group 16 elements) with reference of following: (i) metallic (non-metallic character), (ii) catenation, (iii) thermal stability of hydrides, (iv) oxidation states (v) allotropy.
i) metallic (non- metallic character) of group 16:
In group 16 metallic character increase down the group. This is due to the fact that the electrons become easier to lose as the atomic radius increases. The increase in atomic radius decreases attraction between the positive nucleus and the negative electrons, causing the electrons to be held more loosely.
ii) Catenation property:
In this group sulphur has strong tendency of catenation. Oxygen also shows this tendency to a limited extent.
iii) Thermal stability of hydrides:
The thermal stability decreases from H2O to H2Po because the size of the central atom (from O to Po) increases resulting in longer and weaker M – H bond consequently the bond strength decreases. This results in the decrease of the thermal stability.
The elements of Group 16 exhibit a number of oxidation states .The stability of -2 oxidation state decreases down the group. Polonium hardly shows –2 oxidation state. Since electronegativity of oxygen is very high, it shows only negative oxidation state as –2 except in the case of OF2 where its oxidation state is + 2. Other elements of the group exhibit + 2, + 4, + 6 oxidation states but + 4 and + 6 are more common. Sulphur, selenium and tellurium usually show + 4 oxidation state in their compounds with oxygen and + 6 with fluorine. The stability of + 6 oxidation state decreases down the group and stability of + 4 oxidation state increases (inert pair effect).
v) Allotropy: All elements of the group 16 exhibit allotropyOxygen exist as O2 and O3.
sulphur exist as in number of allotropic forms, such as rhombic forms, such as rhombic, monoclnic plastic sulphur.
selenium has two common allotropic forms red and grey (non-metallic )
Tellurium occurs in two allotropic form, crystalline and amorphous
polonium also existin two allotropic from alpha and beta form.
Describe the molecular shapes of the following: (i) SF4, (ii) BrF5, (iii) IF3, (iv) PF5, (v) XeF2.
SF4 have see saw shape.
ii) BrF5 Square pyramidal with asymmetric charge distribution on cental atom.
iii) IF3 has T shape.
iv) PF5 has trigonal bipyramidal shape
v) XeF2 has linear shape 
Give reasons for:
(i) Ozone is more reactive than oxygen.
(ii) An acidified K2Cr2O7 paper on being exposed to SO2 turns green.
(iii) Sulphuric acid never acts as a reducing agent.
(iv) Noble gases are mostly chemically inert.
(v) Nitrogen is fairly inert.
ii) When SO2 gas is passed through acidified potassium dicromate solution the orange colour potassium dichromate disappear and green colour ofchomium sulphate appears.
3SO2 +K2Cr2O7 +H2SO4 ----> K2SO4 +Cr2(SO4)3green colour +H2O
iii) The concentrated sulphuric acid can act both as an acid and as an oxidising agent. The concentrated sulphuric acid gives a hydrogen ion to the halide ion to produce a hydrogen halide. Because this is a gas, it immediately escapes from the system sulphuric acid ionises in two steps.
H2SO4(aq) + H2O(l) → H3O+(aq) + HSO4–(aq)
Ka1 = very large (Ka1 >10)
HSO4–(aq) + H2O(l) → H3O+(aq) + SO42-(aq)
Ka2 = 1.2 × 10–2
The larger value of
Ka1 (Ka1 >10) means that H2SO4 is largely
dissociated into H+ and HSO4–. Greater the value of dissociation constant (Ka), the stronger is the acid.
iv)Noble gases are inert because electronic configuration of noble gas is ns2 np6 they contain a 'stable octet' of electrons in the outermost shell of the atom (valence electrons). This means that the noble gases do not need to react to lose or gain electrons in order to become stable, since scientists have determined that 8 valence electrons is a stable electron configuration.
Describe the following about halogen family (group 17 elements):
(i) Relative oxidising power.
(ii) Relative acidic strength of their hydrides.
(iii) Oxyacids and their relative oxidising ability.
i) Halogen family have ready acceptance of an electron is the reason for the strong oxidising nature of halogens. F2 is the strongest oxidising halogen and it oxidises other halide ions in solution or even in the solid phase.
ii) Halogens combine with hydrogen to form volatile halides of the formula HX.The bond between hydrogen and halogen is covalent in all the cases.
Acidic Strength. All the hydrogen halides act as acids in their aqueous solutions. The acidic strength varies in the order
HF < HCl < HBr < HI
Reason. All the halogen acids ionise to give H+ ion and halide ion, x-.
HX à H+ + X- ; (where X - = F- , Cl- , Br- , I-)
The above order of acidic character can be explained in terms of strength of H-X bonds, which is in the order H-I< H-Br < H-Cl < H-F. Since H-1 bond is weakest, therefore, HI is the strongest acid. On the other hand H-F bond is strongest, hence it is the weakest acid among all the halogen acids.
iii)
|
Oxidation state |
Chlorine |
Bromine |
Iodine |
Oxidizing power Decrease down the group |
|
+1 |
HClO |
HBrO |
HlO |
|
|
+3 |
HClO2 |
— |
— |
|
|
+5 |
HClO3 |
HBrO3 |
HIO3 |
|
|
+7 |
HClO4 |
HBrO4 |
HIO4 |
|
What is the basicity of H3PO4?
The basicity is defined as the number of hydrogen atom replaceable by a base in a particular acid. H3PO4 has three ionizable hydrogen atoms.
Hence, its basicity is 3.
Give reasons for the following observations:
It is necessary to remove CO when ammonia is prepared by Haber's process.
It is necessary to remove CO when ammonia is prepared by Haber's process because CO acts as a poison and adversely affects the activity of iron catalyst, used in the process.
Account for the following:
(i) Acidic character increases from HF to HI.
(ii) There is a large difference between the melting and boiling points of oxygen and sulphur.
(iii) Nitrogen does not form pentahalide.
(i) The acidic strength of the hydrohalic acids increases from HF to HI because the stability of the acids decreases from HF to HI on account of decrease in bond dissociation enthalpy of H-X bond from HF to HI.
(ii) The oxygen exists as a diatomic molecule, O2, while sulphur exists as a polyatomic molecule, S8. Hence, there is a large difference between the melting point and the boiling point of oxygen and sulphur.
(iii) Group 15 elements form pentahalides when they have empty d-orbitals, which are can be used for forming coordinate bonds. Since nitrogen does not have d-orbitals, it cannot form petahalides.(i) Which allotrope of phosphorus is more reactive and why?
(ii) How the supersonic jet aeroplanes are responsible for the depletion of ozone layers?
(iii) F2 has lower bond dissociation enthalpy than Cl2. Why?
(iv) Which noble gas is used in filling balloons for meteorological observations?
(v) Complete the equation: XeF2 + PF5 →
i) White phosphorus is most reactive of all the allotropes of phosphorus because it is unstable due to the angular strain on P4 molecule with the bond angle of 60°.
(ii) Nitrogen oxide emitted from the exhausts of supersonic jet aeroplanes readily combine with ozone to form nitrogen dioxide and diatomic oxygen.
NO(g) + O3(g) →NO2(g) +O2(g)
Since supersonic jets fly in the stratosphere near the ozone layer, they are responsible for the depletion of ozone layer.
(iii) The size of a fluorine atom is very small as compared to a chlorine atom. Therefore, the repulsion between electrons in the outer most shell of the two atoms in a fluorine molecule is much greater than that in a chlorine molecule. Hence, it requires less energy to break up the fluorine molecule, making its bond dissociation energy lesser than that of chlorine molecule.
(iv) Helium, being light, non-inflammable and unreactive, is used for filling of balloons for metrological observations.
(v) XeF2 + PF5→ [XF]++[PF6]-
Complete the following chemical equations:
(i) Ca3P2 + H2O -->
(ii) Cu + H2SO4(conc.)-->
The balanced reactions are given below:
(i) Ca3P2 + H2O---> 3Ca(OH)2+2PH3
(ii) Cu + H2SO4(conc.) ----> CuSO4+SO2+2H2O
Arrange the following in the order of the property indicated by each set:
(i) HF, HCl, HBr, HI - increasing bond dissociation enthalpy.
(ii) H2O, H2S, H2Se, H2Te- increasing acidic character.
i) The arrangement of the given hydrogen halides in increasing order of bond-dissociation enthalpy is given below:
H-I < H-Br < H-Cl < H-F
(ii) The increasing order of acidic character of the given hydrides of Group 16 elements is given below:
H2O, <H2S,< H2Se,< H2Te
Structural difference between White P and Red P:
|
White P |
Red P |
|
It consists of four P atoms, linked with one another to give rise to a tetrahedral shape. |
It has a polymeric structure, consisting of chains of P4 tetrahedral units that are linked together. |
|
Structure:
|
Structure:
|
Account for the following:
(i) PCl5 is more covalent than PCl3.
(ii) Iron on reaction with HCl forms FeCl2 and not FeCl3.
(iii) The two O-O bond lengths in the ozone molecule are equal.
(i) Greater the positive oxidation state of the central metal atom, greater is its polarising power and thus more is the covalent character of the bond formed between the central metal atom and other atoms.
In PCl5, the central metal atom, P is in +5 oxidation state, while in PCl3, it is in +3 oxidation state. Therefore, PCl5 is more covalent than PCl5.
(ii) Iron reacts with hydrochloric acid in the following manner, resulting in the release of dihydrogen gas.
Fe(s) + 2HCl(aq) ---> FeCl2 (aq) + H2 (g)
The liberated dihydrogen gas may react with the available oxygen and gets converted to a water molecule. This diminishes the chances of oxidation of ferrous chloride to ferric chloride. As a result, FeCl3 is not formed.
(iii) In ozone, the three oxygen atoms are arranged to form a bent shaped structure. The central oxygen atom makes a single bond with one of the terminal oxygen atoms and a double bond with the other terminal oxygen atom. But the electrons of the double bond are delocalised over all the three oxygen atoms. Due to which the single and the double bond are not entirely pure but are the resonance hybrids of single and double bond respectively, giving rise to the O-O bond distance as the average bond distance of the single and double bond.
The resonance structure of the ozone is given below:

How do you prepare:
(i) K2MnO4 from MnO2?
(ii) Na2Cr2O7 from Na2CrO4?
i) K2MnO4 can be prepared from pyrolusite (MnO2). The ore is fused with KOH in the presence of either atmospheric oxygen or an oxidising agent, such as KNO3 or KClO4, to give K2MnO4.
2 MnO2 + 4 KOH + O2 ![]() 2 K2MnO4 +2H2O
2 K2MnO4 +2H2O
green
ii) Na2Cr2O7 can be prepared from Na2CrO4 in the following way:
For the preparation of sodium dichromate, the yellow solution of sodium chromate is acidified with sulphuric acid to give a solution from which orange sodium dichromate, Na2Cr2O7.2H2O can be crystallised.
Balanced equation for above reactions is as follows:
2 Na2CrO4 + 2H+ ---> Na2Cr2O7 + 2 Na+ + H2O
Account for the following:
(i) Mn2+ is more stable than Fe2+ towards oxidation to +3 state.
(ii) The enthalpy of atomization is lowest for Zn in 3d series of the transition elements.
(iii) Actinoid elements show a wide range of oxidation states.
i) Electronic configuration of Mn2+ is [Ar]183d5
Electronic configuration of Fe2+ is [Ar]18 3d6
It is known that half-filled and fully-filled orbitals are more stable. Therefore, Mn in +2 state has a stable d5 configuration. Therefore, Mn2+ shows resistance to oxidation to Mn3+. Also,
e2+ has 3d6 configuration and by losing one electron, its configuration changes to a more stable 3d5 configuration.
Therefore, Fe2+ gets oxidised to Fe3+ easily.
ii) The extent of metallic bonding an element undergoes, decides the enthalpy of atomisation.
The more extensive the metallic bonding of an element, the more will be its enthalpy of atomisation. In all transition metals (except Zn, electronic configuration: 3d10 4s2), there are some unpaired electrons that account for their stronger metallic bonding. Due to the absence of these unpaired electrons, the inter-atomic electronic bonding is the weakest in Zn and as a result, it has the least enthalpy of atomisation.
iii) Actinides exhibit larger oxidation states because of very small energy gap between 5f, 6d and 7s sub-shells. The energies are calculated on the basis of (n+l) rule. The (n+l) values of the three orbitals are:
5 f = 5 + 3 = 8
6 d = 6 + 2 = 8
7 s = 7 + 0 = 7
Since, all the values are almost same, therefore all orbitals can involve in bonding resulting in larger oxidation number for actinoids.
(i) Name the elements of 3d transition series that show a maximum number of oxidation states. Why does this happen?
(ii) Which transition metal of 3d series has positive E0 (M2+/M) value and why?
(iii) Out of Cr3+ and Mn3+, which is a stronger oxidising agent and why?
(iv) Name a member of the lanthanoid series that is well-known to exhibit +2 oxidation state.
(v) Complete the following equation: MnO4- + 8H+ + 5e- -->
1) In 3d-series of transition metals, manganese has an atomic number of 25 that gives the electronic configuration as [Ar] 3d54s2 ,where we see that the maximum number of unpaired electrons is found in manganese atom; so, it can show a maximum oxidation state upto +7.
2) Copper is the transition metal of 3d series that exhibits positive E0 (M2+/M). The value of E0(M2+/M) for copper is (+0.34). This happens because the E0 (M2+/M) value of a metal depends on the energy changes involved in the following:
(i) Sublimation energy: The energy required for converting one mole of an atom from the solid state to the gaseous state.
M(s)--> M(g) ![]() sH (Sublimation energy)
sH (Sublimation energy)
(ii) Ionisation energy: The energy required to take out electrons from one mole of atom in the isolated gaseous state.
M(g)--> M2+(g) ![]() iH (Ionisation energy)
iH (Ionisation energy)
(iii) Hydration energy: The energy released when one mole of ions are hydrated.
M2+(g)--> M2+(aq) ![]() hydH (Hydration energy)
hydH (Hydration energy)
Since, copper has a high energy of atomization and low hydration energy, the E0(M2+/M) value for copper is positive.
3) Out of Cr3+ and Mn3+, Mn3+ is a stronger oxidising agent because it has 4 electrons in its valence shell and when it gains one electron to form Mn2+, it results in the half-filled (d5) configuration that has extra stability.
4) Europium (Eu) is well-known to exhibit +2 oxidation state due to its half filled f orbital in +2 oxidation state.
5) MnO4- + 8H+ + 5e- ---> Mn2+ + 4 H2O
What is the covalency of nitrogen in N2O5?
In N2O5, the covalency of N is restricted to 4 due to sp2 hybridization of nitrogen atom involving one 2s and three 2p orbitals.
Arrange the following in increasing order of their basic strength in aqueous solution:
CH3NH2, (CH3)3N, (CH3)2NH
With the increase in the alkyl group, the +I effect will increase which will increase the ease of donation of lone pair electron. But in water one other factor is controlling the strength of basicity.
Amine will accept a proton and from cation will be stabilised in water by salvation (by hydrogen bonding) better the salvation by hydrogen bonding higher will be the basic strength.
(CH3)3N<CH3NH2< (CH3)2NH
What happens when
(i) PCl5 is heated?
(ii) H3PO3 is heated?
Write the reactions involved ?
(i) All the bonds that are present in PCl5 no similar. It has three equatorial and two axial bond, the equatorial bonds are stronger than axial one, therefore when PCl5 is heated strongly.
It decomposes to form: PCl5 ![]() PCl3 + Cl2
PCl3 + Cl2
(ii) H3PO3, on heating, undergoes disproportionation reaction to form PH3 and H3PO4. The oxidation numbers of P in H3PO3, PH3, and H3PO4 are +3, −3, and +5 respectively. As the oxidation number of the same element is decreasing and increasing during a particular reaction, the reaction is a disproportionation reaction.
Give reasons for the following:
(i) Bond enthalpy of F2 is lower than that of Cl2.
(ii) PH3 has lower boiling point than NH3(i) Bond enthalpy of F2 is lower than that of Cl2 because F atom is small in size and due to this the electron-electron repulsions between the lone pairs of F-F are very large. Thus, the bond dissociation energy of F2 is lower than that of Cl2.
(ii) PH3 has a lower boiling point than NH3 because NH3 molecule possesses intermolecular hydrogen bonding which binds them strongly whereas PH3 has weaker Vander Waal’s forces. Thus, PH3 has a lower boiling point than NH3.
Draw the structures of the following molecules:
(i) BrF3
(ii) (HPO3)3
(iii) XeF4
The structures of following molecules are as follows:
(i) BrF3, Bent T-shape
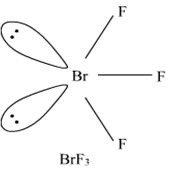
ii) (HPO3)3, cyclic structure
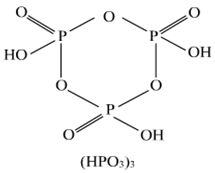
iii) XeF4, Square planar
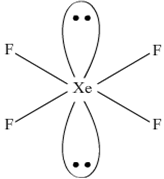
Account for the following:
(i) Helium is used in diving apparatus.
(ii) Fluorine does not exhibit positive oxidation state.
(iii) Oxygen shows catenation behaviour less than sulphur
(i) Helium mixed with oxygen under pressure is given to sea-divers for respiration. However, pure oxygen can be toxic at great concentrations at the depth. Therefore oxygen can be mixed with helium to reduce oxygen concentration while eliminating nitrogen. During controlled decompression, the helium would also diffuse out of tissue and the lungs are more easily than nitrogen avoiding the bends .Use of helium for relatively shallow scuba diving would likely permit longer driving times with less threat of the bends.
(ii) Fluorine being the most electronegative atom does not exhibit positive oxidation state because the electrons in fluorine are strongly attracted by the nuclear charge because of the small size of a fluorine atom and therefore, removal of an electron is not possible.
(iii) Sulphur shows catenation behaviour more than that of oxygen because the oxygen atom is smaller in size as compared to sulphur, the O-O bonds in oxygen experiences repulsions due to the lone pairs present on oxygen atom and therefore, are weaker as compared to the S-S bonds.
Draw the structures of the following molecules:
(i) XeF2
(ii) H2S2O8XeF2, Linear
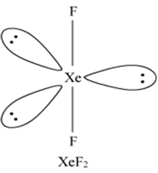
ii) H2S2O8
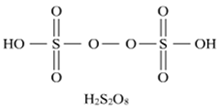
Which one of ![]() is not likely to exist and why?
is not likely to exist and why?
The oxidation state of P in ![]() is +5 while in
is +5 while in ![]() is +3. As we know that the stability of the +5 oxidation state is highest on top of the group and decrease down the group and stability of +3 is low on the top of the group and increase down the group.
is +3. As we know that the stability of the +5 oxidation state is highest on top of the group and decrease down the group and stability of +3 is low on the top of the group and increase down the group.
Therefore ![]() is more likely to exist.
is more likely to exist.
Arrange the following in the decreasing order of their basic strength in aqueous solutions:
CH3NH2, (CH3)2NH, (CH3)3 N and NH3
With the increase in alkyl group, the +I effect will increase which will increase the ease of donation of lone pair electron .But in water one other factor is controlling the strength of basicity. Amine will accept a proton and from cation will be stabilised in water by salvation (by hydrogen bonding).better the salvation by hydrogen bonding higher will be the basic strength.
(CH3)2 NH > CH3 NH2 > (CH3)3 N > NH3
(a) Complete the following chemical reactions equations:
(i) P4+SO2Cl2 -->
(ii) XeF6+H2O -->
(b) Predict the shape and the asked angle (90° or more or less) in each of the following cases:
(i) and the angle O - S - O
(ii) ClF3 and the angle F - Cl - F
(iii) XeF2 and the angle F - Xe - F
(i) P4+ 10SO2Cl2 ---> 4 PCl5+ 10SO2
(ii) Equation for complete hydrolysis:
XeF6+H2O ---->XeO3+6HF
Equations for partial hydrolysis
XeF6+H2O ---> XeOF3+2HF
XeF6+2H2O ---> XeO2F2+4HF
b) (i)
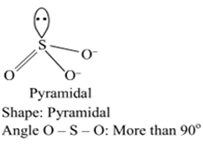
ii)
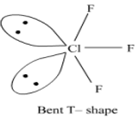
Angle F – Cl - F: Less than90o
iii)
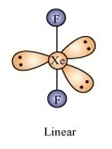
Angle F- Xe- F 80o
Complete the following chemical equations:
(i) NaOH+Cl2 -->
(ii) XeF4+O2F2--->
(b) Draw the structures of the following molecules:
(i) H3PO2
(ii) H2S2O7
(iii) XeOF4
a) (i) 6NaOH +3Cl2 -----> 5NaCl+NaClO3+3H2O
(hot and conc.)
(ii) XeF4+O2F2-----> XeF6+ O2
b)
i)
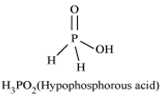
ii)
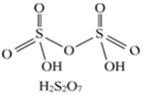
iii)
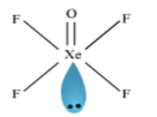
State reasons for each of the following:
The N-O bond in is shorter than the N-O bond in
The shorter N - O bond in is due to the existence of resonance in. The resonating structures can be drawn as follows.

Due to resonance in, the two bonds are equivalent. This leads to a decrease in bond length.
Thus, the N - O bond length in resembles a double bond.
Now, the resonating structures for can be drawn as:
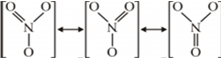
As seen from the above resonating structures of ![]() , the three oxygen atoms are sharing two single bonds and one double bond. So, the real N - O bond length resembles a single bond closely.
, the three oxygen atoms are sharing two single bonds and one double bond. So, the real N - O bond length resembles a single bond closely.
In ![]() the lone pair is delocalized between the 2 oxygen groups. Bond order equal to 1+1/2=3/2.
the lone pair is delocalized between the 2 oxygen groups. Bond order equal to 1+1/2=3/2.
In ![]() lone pair shared between three oxygen atom hence bond order =1+1/3=4/3.
lone pair shared between three oxygen atom hence bond order =1+1/3=4/3.
Greater the bond order shorter the bond. Hence bond length of ![]() less than that of
less than that of ![]()
This explains the existence of shorter bond length of the N - O bond ![]() in than in
in than in ![]() .
.
State reasons for each of the following:
SF6 is kinetically an inert substance.
The kinetic inertness of SF6 can be explained on the basis of its structure.
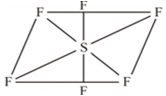
The six fluoride (F-) atoms protect the sulphur atom from attack by the regents to such an extent that even thermodynamically most favorable reactions like hydrolysis do not occur.
State reasons for each of the following:
(i) All the P-Cl bonds in PCl5 molecule are not equivalent.
(ii) Sulphur has a greater tendency for catenation than oxygen.
(i) In gaseous and liquid state, PCl5 has a trigonal bipyramidal structure. In this structure, the two axial P-Cl bonds are longer and less stable than the three equatorial P-Cl bonds. This is because of the greater bond pair - bond pair repulsion in the axial bonds. Hence, all the bonds in PCl5 are not equivalent.
(ii) Because of stronger S-S bonds as compared to O-O bonds, sulphur has a greater tendency for catenation than oxygen.
(i) NF3 is an exothermic compound whereas NCl3 is not.
(ii) F2 is most reactive of all the four common halogens.
(b) Complete the following chemical equations:
(i) C + H2SO4 (conc.)-->
(ii) P4 + NaOH + H2O-->
(iii) Cl2+F2 ------>
(excess)
(i) As we move down the group 17, the size of the atom increases from fluorine to chlorine. The larger difference in the size of N and Cl results in the weakness of strength of N-Cl bond.
On the other hand, the difference in size of N and F is small; consequently, the N-F bond is quite strong. As a result, NF3 is an exothermic compound.
(ii)
1. F-F bond has low enthalpy because the fluorine atom has a small size and due to their small size, there is repulsion between two atoms making its bond enthalpy lower, hence more reactivity is more.
2. It has a small size and high charge density due to which it is the most electronegative element.
(a) Account for the following:
(i) The acidic strength decreases in the order HCl > H2S > PH3
(ii) Tendency to form pentahalides decreases down the group in group 15 of the periodic table.
(b) Complete the following chemical equations:
(i) P4 + SO2Cl2-->
(ii) XeF2 + H2O--->
(iii) I2+HNO3(conc.)--->
(i) The acidity of a molecule depends on the polarity of the bond between central atom and the hydrogen atom. Greater the polarity higher will be the acidity.
And the polarity of the bond depends on the electronegativity of the central atom. In a period, the electronegativity decreases in the order Cl > S > P. As a result, the loss of H+ ions decreases.
Thus, the acidic strength of the hydrides decreases in the
Following order:
HCl > H2S > PH3
(ii) Nitrogen does not form pentahalide because it does not have d-orbital. P, As, Sb form pentahalide. Bi does not form pentahalide. The tendency to form pentahalide decrease down the group. This because of inert pair effect.
Due to the inert pair effect, ns2 electron remains inert in a chemical reaction and element shows -2 oxidation state. Inert pair effect increases down the group. Thus the tendency to form pentahalides decrease down the group 15.
(b)
(i) P4 + 10SO2Cl2---> 4PCl5 + 10SO2
(ii) 2XeF2 + 2H2O----> 2Xe + 4HF + O2
(iii) I2 + 10HNO3--> 2HIO3+10NO2+4H2O
Write the structures of the following molecules:
(i) H2SO3
(ii) XeOF4
|
|
Molecules |
Structures |
|
(i) |
H2SO3 |
 |
|
(ii) |
XeOF4 |
|
Arrange the following in increasing order of their basic strength:
(i) C6H5 – NH2, C6H5 – CH2 – NH2, C6H5 – NH – CH3

(i) C6H5-NH2 < C6H5-NH-CH3 < C6H5-CH2-NH2
Reason:
C6H5-NH2 will be least basic because of the delocalization of the lone pair of electrons present on the N-atom over the benzene ring due to the ‒R effect of the C6H5 group. However, C6H5-CH2-NH2 will be more basic than C6H5-NH-CH3 because of the electron-releasing nature of the CH3- a group that increases the electron density on the N-atom, making the lone pair of electrons on the N-atom easily available for donation to a proton. The basicity of C6H5-NH-CH3 will be intermediate of C6H5-NH2 and C6H5-CH2-NH2 because the C6H5- the group will tend to pull the electron density from the N-atom. On the other hand, the CH3- group will tend to increase the electron density on the N-atom. Thus, the basic strength of the given amines will follow the above-mentioned order.
(ii) 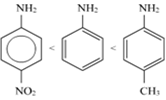
Reason:
The -CH3 group will increase the electron density on the benzene ring because of it is +I effect, while the NO2 group will decrease the electron density on the benzene ring because of its ‒I effect. Hence, the C6H5-NH2 molecule having - the CH3 group will be more basic than C6H5-NH2. Also, it will be more basic than the C6H5-NH2 molecule with the NO2 group.
Give reasons for the following:
(i) N2 is less reactive at room temperature.
(ii) H2Te is the strongest reducing agent amongst all the hydrides of Group 16 elements.
(iii) Helium is used in diving apparatus as a diluent for oxygen.
Two nitrogen atoms are joined by triple bonds. The nitrogen atom is very small, therefore the bond length is also quite small (109.8pm) and as the result, the bond dissociation energy is quite high (946Kj/mol) Therefore, N2 is less reactive at room temperature.
H2Te is the strongest reducing agent among the hydrides of group 16. The size of Te is very large due to which the bonding between hydrogen and Te is not strong. On the other hand, the electronegativity of Te is very less. So it will easily loose hydrogen. As the size of the elements increases in the order O < S < Se < Te, thus bond strength decreases from H2O to H2Te and therefore, the bond dissociation enthalpy decreases. Hence, due to the increase in the tendency to release proton, the element's reducing tendency also increases.
(iii) Helium is used in diving apparatus as a diluent for oxygen because it is chemically inert and does not participate in the chemical reaction. Helium has low solubility in water than many other gases, such as nitrogen. Due to low solubility means it does not enter the bloodstream, even under pressure commonly experienced by deep sea divers.
Draw the structures of the following molecules:
(i) XeF6
(ii) H2S2O7
(i) Structure of XeF6 :

(ii) H2S2O7

Give reasons for the following:
(i) Oxygen is a gas but sulphur is solid.
(ii) O3 acts as a powerful oxidising agent.
(iii) BiH3 is the strongest reducing agent amongst all the hydrides of Group 15 elements.
(i) Oxygen forms O2 which is a gas and sulphur forms S8 which is solid this can be explained as:
Due to the small size of oxygen, it has less tendency for catenation and the high tendency of pp-pp multiple bonds, hence forms stable O2 molecules whereas sulphur because of its higher tendency for catenation and lesser tendency to form pp-pp multiple bonds forms S8 molecules having 8-membered puckered ring. Held together by strong covalent bonds and exist as a polyatomic molecule, so it exists solid.
(ii) Ozone is not a very stable compound under normal conditions and decomposes readily on heating to give a molecule of oxygen and nascent oxygen. Nascent oxygen, being a free radical, is very reactive.
![]()
Therefore, O3 acts as powerful oxidising agent.
(iii) BiH3 is the strongest reducing agent amongst all the hydrides of group-15 elements because as we more down the group, the atomic size increases and the stability of the hydrides of group 15 element decreases. Since the stability of hydrides decreases on moving from NH3 to BiH3, the reducing character of the hydrides increases on moving from NH3 to BiH3.
What is the basicity of H3PO2 acid and why?
Basicity of H3PO2 depends on upon the number of ionizable -OH groups present in the molecule. That is the number of hydrogen attached to the electronegative atom oxygen.
H3PO2 has one ionizable –OH group, thus its basicity is 1.
The structure of H3PO2 is as follows:

Explain the following facts giving appropriate reason in each case:
(i) NF3 is an exothermic compound whereas NCl3 is not.
(ii) All the bonds in SF4 are not equivalent.
(i) As we move down the group 17, the size of the atom increases from fluorine to chlorine. The instability of NCl3 is due to the weak NCl bond. This is due to the large difference in the size of nitrogen and chlorine atoms. On the other hand, atoms of both nitrogen (75 pm) and fluorine (72 pm) are small sized. Thus, bonding in NF3 is quite strong and it is an exothermic compound.
(ii)SF4 has four bonded atoms and one lone pair so SF4 has sp3d hydridisation and thus have trigonal bipyramid structure in which one axial position is occupied by a lone pair of electrons. This lone pair finds a position that minimizes the number of 900 repulsion it has with bonding electron pairs. This results in two types of angles.
Equatorial bond angle F - S - F° (LP-BP repulsion >BP – BP repulsion)
Axial bond angle F - S - F<90°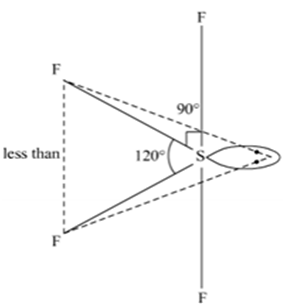
(a) Draw the molecular structure of the following compounds.
(i) N2O5
(ii) XeOF4
(b) Explain the following observation:
(i) Sulphur has a greater tendency for catenation than oxygen.
(ii) ICI is more reactive than I2.
(iii) Despite the lower value of its electron gain enthalpy with a negative sign, fluorine (F2) is a stronger oxidizing agent than Cl2.
i)

ii)
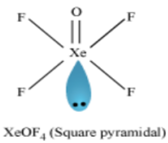
(b)
(i) Due to the small size of oxygen, it has less tendency for catenation and the high tendency of pp-pp multiple bonds, hence forms stable O2 molecules whereas sulphur because of its higher tendency for catenation and lesser tendency to form pp-pp multiple bonds forms S8 molecules having 8-membered puckered ring.
(ii)Inter -halogen bonds are weaker (it is between two different halogen like ICl) because of its partly ionic character due to the difference in electronegativity.
While when the same halogen forms X2 molecules like I2. They form covalent bonds which are stronger than interhalogen compound and weak bond obviously is more reactive than the stronger bond and that’s why ICl is more reactive than I2.
(iii) Fluorine is a much stronger oxidising agent than chlorine. The oxidising power depends on three factors.
1. Bond dissociation energy
2. Electron gains enthalpy
3. Hydration enthalpy
The electron gain enthalpy of chlorine is more negative than that of fluorine.
However, the bond dissociation energy of fluorine is much lesser than that of chlorine. Also, because of its small size, the hydration energy of fluorine is much higher than that of chlorine. Therefore, the latter two factors more than compensate for the less negative electron gain enthalpy of fluorine. Thus, fluorine is a much stronger oxidising agent than chlorine.
(a) Complete the following chemical equation
(i) Cu + HNO3 (dilute) --->
(ii) XeF4 + O2F2 -->
(b) Explain the following observation:
(i) Phosphorus has a greater tendency for catenation than nitrogen.
(ii) Oxygen is a gas but sulphur a solid.
(iii) The halogens are coloured. Why?
(i) 3Cu + 8HNO3(dilute) ----> 3Cu (No3)2 + 2NO + 4H2O
(ii) XeF4 + O2F2 ---> XeF6 + O2
(b)
(i)Catenation is the property of atoms of an element to link together and in case of nitrogen the size of it being small and due to electron repulsion it can exist stably in diatomic form
Catenation is much more common in phosphorous compounds than in nitrogen compounds. This is because of the relative weakness of the N-N single bond as compared to the P-P single bond. Since nitrogen atom is smaller, there is greater repulsion of electron density of two nitrogen atoms, thereby weakening the N-N single bond.
(ii) Oxygen forms O2 which is a gas and sulphur forms S8 which is solid this can be explained as:
Due to the small size of oxygen, it has less tendency for catenation and the high tendency of pp-pp multiple bonds, hence forms stable O2 molecules whereas sulphur because of its higher tendency for catenation and lesser tendency to form pp-pp multiple bonds forms S8 molecules having 8-membered puckered ring. Held together by strong covalent bonds and exist as polyatomic molecule, so it exists solid
(iii) All the halogens possess a valence shell electronic configuration s2, p5. This means that they contain unpaired electrons in their outermost p- orbital. These electrons absorb light and get promoted to higher orbitals. When they return to their ground state, they emit radiation which falls in the visible region of electromagnetic spectrum. Hence appear.
(a) Account for the following:
(i)Ozone is thermodynamically unstable.
(ii)Solid PCl5 is ionic in nature.
(iii)Fluorine forms only one oxoacid HOF.
(b) Draw the structure of
(i) BrF5
(ii) XeF4
OR
(i)Compare the oxidizing action of F2 and Cl2 by considering parameters such as bond dissociation enthalpy, electron gain enthalpy and hydration enthalpy.
(ii)Write the conditions to maximize the yield of H2SO4 by contact process.
(iii)Arrange the following in the increasing order of property mentioned:
(a)H3PO3, H3PO4, H3PO2 (Reducing character)
(b)NH3, PH3, AsH3, SbH3, BiH3 (Base strength)
a)
(i) Ozone decomposes into O2 with the evolution of heat, i.e. ΔH is negative (exothermic).
O3 --> O2 + O
ΔH = negative
Since the decomposition of O3 increases the number and freedom of particles, entropy also increases.
Therefore, DS = positive Now, ΔG = ΔH – TΔS
Both −∆H and
-T∆S(since ∆S is positive) result into large negative ∆G. Hence, O3 becomes thermodynamically unstable and decomposes into oxygen easily.
(ii) PCl5 is ionic in solid state because it exists as [PCl4]+ [PCl6]− in which the cation has tetrahedral geometry and the anion has octahedral geometry.
(b) (i) BrF5
(ii) XeF4
Or
(i) Although electron gain enthalpy of fluorine is less than that of chlorine because of the small size of fluorine, but the oxidising power depends on other factors like bond dissociation energy and hydration energy. The smaller the size of the atom, the greater the hydration enthalpy. Fluorine being small in size has higher hydration enthalpy as compared to chlorine.
Also, fluorine faces greater inter-electronic repulsion among its lone pairs of electrons because of its small size, while there is very less repulsion in chlorine. Hence, the bond dissociation enthalpy of fluorine is lower than that of chlorine.
Thus, the high hydration enthalpy and low bond dissociation enthalpy of fluorine result in its higher oxidising power as compared to that of chlorine.
(ii) Manufacturing of sulphuric acid via the contact process involves three steps:
(1) Burning of ores to form SO2
(2) Conversion of SO2 to SO3 using V2O5 as a catalyst
(3) Absorption of SO3 in H2SO4 to give oleum
The second step, i.e. conversion of SO2 to SO3 is the key step. Since this reaction is exothermic in nature and two moles of gaseous reactant give one mole of gaseous
Pb(NO3)2 on heating gives a brown gas which undergoes dimerization on cooling ? Identify the gas.
Nitrogen dioxide gas evolved.
Write the structures of the following:
i) BrF3
ii) XeF4
Or
What happens when:
i) SO2 gas is passed through an aqueous solution Fe3+ salt ?
ii) XeF4 reacts with SbF5 ?
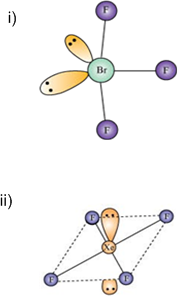
Or
(i) 2Fe3+ + SO2 + 2H2O ---> 2Fe2+ + SO42- + 4H+
(ii) XeF4 + SbF5 ---->[ XeF3]+ [SbF6]-
Give reasons:
i) SO2 is reducing while TeO2 is an oxidizing agent.
ii) Nitrogen does not form pentahalide.
iii) ICl is more reactive than I2
i) Sulphur dioxide is reducing agent because sulphur has d-orbital so it can easily expand its oxidation state +4 to +6 and thus behave as reducing agent.
in the case of TeO2, Te is a heavier element and due to inert pair effect, the Te does expand its oxidation state +4 to +6.
ii) The electronic state of Nitrogen is 1s2 2s2 2p3. Nitrogen does not have d orbital and due to the absence of d -orbital it does not form pentahalide.
iii) ICl is more reactive than I2 because I-Cl bond in ICl is weaker than I-I bond in I2.
i) Name the method of refining of nickel
ii) what is the role of cryolite in the extraction of aluminium
iii) what is the role of limestone in the extraction of iron from its oxides ?
(i) Mond’s Process use in the refining of nickel
(ii)The melting point of alumina is very high. It is dissolved in cryolite which lowers the melting point and brings conductivity / acts as a solvent.
(iii)Limestone is decomposed to CaO ,which removes silica impurity of the ore as slag.
The elements of 3d transition series are given as:
Sc Ti V Cr Mn Fe Co Ni Cu Zn
Answer the following:
i) Write the element which shows a maximum number of oxidation states.Give reason.
ii) Which element has the highest m.p?
iii) Which elements shows only +3 oxidation state ?
iv) which element is a strong oxidising agent in +3 oxidation state and why?
i) Mn. It has maximum unpaired electrons.
ii) Cr
iii) Sc
iv) Manganese. Mn3+ to Mn2+ results in the stable half filled (d5) configuration.
How would you account for the following?
(i) NF3 is an exothermic compound but NCl3 is not.
(ii) The acidic strength of compounds increases in the order:
PH3 < H2S < HCl
(iii) SF6 is kinetically inert.(i) As we move down the group 17, the size of the atom increases from fluorine to chlorine. The larger difference in the size of N and Cl results in the weakness of strength of N - Cl bond. On the other hand, the difference in size of N and F is small; consequently the N -F bond is quite strong. As a result, NF3 is an exothermic compound.
(ii) In a period, the electronegativity decreases in the order Cl > S > P. As a result, the loss of H+ ions decreases. Thus, the acidic strength of the hydrides decreases in the following order. HCl > H2S > PH3
(iii) The kinetic inertness of SF6 can be explained on the basis of its structure.

(a) Draw the structures of the following molecules:
(i) (HPO3)3
(ii) BrF3
(b) Complete the following chemical equations:
(i) HgCl2 + PH3-->
(ii) SO3 + H2SO4 -->
(iii) XeF4 + H2O -->
OR
(a) What happens when?
(i) Chlorine gas is passed through a hot concentrated solution of NaOH?
(ii) Sulphur dioxide gas is passed through an aqueous solution of a Fe (III) salt?
(b) Answer the following:
(i) What is the basicity of H3PO3 and why?
(ii) Why does fluorine not play the role of a central atom in inter-halogen compounds?
(iii) Why do noble gases have very low boiling points?
(i) (HPO3)3
(ii) BrF3 has a bent T-shape and can be drawn as follows.
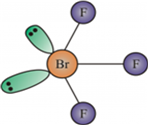
(b)
(i) 3 HgCl2 + 2 PH3 --> Hg3P2 + 6 HCl
(ii) SO3 + H2SO4 ---> H2S2O7
(iii) 6 XeF4 +12 H2O ---> 4Xe + 2 XeO3 + 24 HF + 3O2
Or
(a)
(i) When chlorine is passed through a hot concentrated solution of NaOH, it undergoes disproportionation in which chlorine is simultaneously reduced to Cl-and is oxidised to
3Cl2 + 3NaOH --> 5NaCl + NaClO3 + 3H2O
(ii) SO2 acts as a reducing agent when passed through an aqueous solution containing Fe(III) salt. It reduces Fe(III) to Fe(II) i.e., ferric ions to ferrous ions.
2Fe3+ + SO2 + 2H2O ---> 2Fe2+ + SO42-+ 4H+
(b)
(i) Basicity of H3PO3 depends upon the number of ionizable -OH groups present in the molecule. That is the number of hydrogen attached to the electronegative atom oxygen.
H3PO3 has two ionizable –OH group, thus its basicity is 2
The structure of H3PO3 follows as:
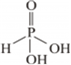
(ii) Fluorine does not play the role of a central atom in inter-halogen compounds because of the absence of d- orbital. Also, because of the small size of fluorine, it cannot accommodate larger halogen groups.
(iii) The noble gases have low boiling point due to the fact that the noble gases are mono atomic. Having no interatomic forces except weak dispersion forces.
What is the basicity of H3PO3?
H3PO3 ionises to give two H+ ions. Thus, it is dibasic in nature.
H3PO3 --> 2H+ + HPO2-3
Complete the following equations:
(i) P4 + H2O →
(ii) XeF4 + O2F2 →
(i) P4 + H2O → No reaction
(ii) XeF4 + O2F2 → XeF6 + O2
Give reasons for the following:
(i) (CH3)3P = O exists but (CH3)3N = O does not.
(ii) Oxygen has less electron gain enthalpy with the negative sign than sulphur.
(iii) H3PO2 is a stronger reducing agent than H3PO3.
(i) N atom cannot expand its covalency beyond four due to the absence of vacant d- orbitals, whereas P atom possesses vacant d- orbitals. As a result, (CH3)3P = O exists but (CH3)3N = O does not.
(ii) Due to the small size and compact nature of the oxygen atom, the incoming electron is not accommodated with ease. As a result, oxygen has less electron gain enthalpy with a negative sign than sulphur.
(iii) Greater the number of element−hydrogen (E−H) bonds present in a compound, greater is the reducing nature of the compound. H3PO2 has two P−H bonds while H3PO3 has one P−H bond. Thus, H3PO2 is a stronger reducing agent than H3PO3.
What happens when
H3PO3 is heated?
Phosphorous acid H3PO3, on heating, undergoes disproportionation reaction to form Phosphine (PH3) and Phosphoric acid (H3PO4)
4H3PO3 +heat → 3H3PO4 +PH3
Give reasons:
Thermal stability decreases from H2O to H2Te.
The thermal stability of the hydrides of group 16 elements decreases down the group, i.e., H2O > H2S > H2Se > H2Te > H2Po. This is because M-H bond dissociation energy decreases down the group with the increase in the size of a central atom.
Give reasons:
Fluoride ion has higher hydration enthalpy than chloride ion.
The size of fluoride ion is small as compared to the chloride ion. Hence, when the two are dissolved in water, the energy of hydration released in the case of fluoride ion will be more than chloride ion due to stronger interactions (ion-dipole) between the ion and the water molecules.
Give reasons:
Nitrogen does not form pentahalide.
Nitrogen does not form pentahalide because it can not extend its valency upto 5 due to unavailability of d orbitals.
How is the variability in oxidation states of transition metals different from that of the p-block elements?
In p block elements the difference in oxidation state is 2 and in transition
metals the difference is 1.
Among the hydrides of Group-15 elements, which have the
- Lowest boiling point?
- Maximum basic character?
- Highest bond angle?
- Maximum reducing character?
- PH3 has a lowest boiling point
- NH3 has a maximum basic character
- NH3 has the highest bond angle
- BiH3 has maximum reducing character
Give reasons:
H3PO3 undergoes disproportionation reaction but H3PO4 does not.
H3PO3 undergoes disproportionation reaction but H3PO4 does not. This is because in H3PO3, P is in +3 oxidation state which can get oxidised as well as reduced. Thus, H3PO3 gives disproportionation, reaction as:
On the other hand, in H3PO4, P is in its maximum oxidation state of +5, which can duly be reduced but not oxidised further. So, H3PO4 does not show disproportionation.
Give Reason:
Dioxygen is a gas while Sulphur is a solid at room temperature.
Oxygen is smaller in size and thus due to its small size complete its octet by forming pπ = p bond. Therefore, O2 is a discrte molecule and the intermolecular forces of attraction are weak vander waals forces. Hence, O2 is a gas. While sulphur is large in size, cannot form p= p bonds. Therefore, in order to gain stability, it exists as S8 which is a solid.
When concentrated sulphuric acid was added to an unknown salt present in a test tube a brown gas (A) was involved. This gas intensified when copper turnings were added to this test tube. On cooling, the gas (A) changed into a colourless solid (B).
(i) Identify (A) and (B)
(ii) Write the structures of (A) and (B)
(iii) Why does gas (A) change to solid on cooling
(i) A is NO2 gas (because NO3- salt reacts with conc. H2SO4 to give NO2 gas which is brown). (B) is N2O4
(ii)
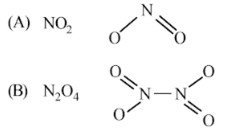
(iii) NO2 (A) is an odd electron-molecule. Thus, to become stable, it decreases to give N2O4 which is a colourless solid.
Arrange the following in the decreasing order of their reducing character:
HF, HCl, HBr, HI
Reducing character depends on the ease of release of H. Down the group, atomic size of halogen increases, bond length increases, bond strength decreases, thus it becomes easier to release H. So, the correct order of reducing character is:
HF<HCl<HBr<HI
The reaction of zinc with dilute and concentrated nitric acid, respectively, produces:
-
NO2 and NO
-
NO and N2O
-
NO2 and N2O
-
N2O and NO2
D.
N2O and NO2
Zn + 4 HNO3 (conc.)→ Zn(NO3)2 + 2H2O + 2NO2
4Zn + 10HNO3 (dil) → 4Zn(NO3)2 + N2O + 5H2O
Which one has the highest boiling point?
-
He
-
Ne
-
Kr
-
Xe
D.
Xe
As, we move down the group of noble gases molecular mass increases by which dipole produced for a moment and hence London forces increases from He to Xe. Therefore more amount of energy is required to break these forces, thus boiling point also increases from He to Xe.
Assertion : Nitrogen and Oxygen are the main components in the atmosphere but these do not react to form oxides of nitrogen.
Reason : The reaction between nitrogen and oxygen requires high temperature.
-
Both assertion and reason are correct, but the reason is not the correct explanation for the assertion
-
Both the assertion and reason are incorrect but the reason is not the correct explanation for the assertion.
-
The assertion is incorrect, but the reason is correct
-
Both assertion and reason are correct, and the reason is the correct explanation for the assertion
A.
Both assertion and reason are correct, but the reason is not the correct explanation for the assertion
Among the following oxoacids, the correct decreasing order of acid strength is
-
HOCl > HClO2>HClO3 > HClO4
-
HClO4>HOCl> HClO2>HClO3
-
HClO4>HClO3>HClO2>HOCl
-
HClO2>HClO4>HClO3>HOCl
C.
HClO4>HClO3>HClO2>HOCl
Decreasing order of strength of oxoacids
HClO4> HClO3>HClO2>HOCl
Reason: consider the structure of conjugate bases of each oxyacid of chlorine.
A negative charge is more delocalised on ClO4- due to resonance, hence ClO4- is more stable (and less basic).
Hence, we can say as the number of the oxygen atom (s) around C|-atom increases as oxidation number of Cl- atom increases and thus, the ability of loose the H+ increases.
The molecule having smallest bond angle is
-
NCl3
-
AsCl3
-
SbCl3
-
PCl3
C.
SbCl3
On moving down the group, the size of the central atom increases and electronegativity decreases. Thus, the bond pairs of electrons tend to lie farther away from the central atoms as we move from N to Sb. Hence, bond pairs repulsion is maximum in NCl3 and minimum in SbCl3. Thus, bond angle decreases from NCl3 (maximum)to SbCl3 (minimum).
Which of the following statement is wrong?
-
The stability of hydrides increases from NH3 to BiH3 in group 15 of the periodic table.
-
Nitrogen cannot form dπ-pπ bond.
-
Single N - N bond is weaker than the single P - P bond.
-
N2O4 has two resonance structure
A.
The stability of hydrides increases from NH3 to BiH3 in group 15 of the periodic table.
The stability of hydrides decreases from NH3 to BiH3 which can be observed from their bond dissociation enthalpy. The correct order is NH3 < PH3 < AsH3 < SbH3 < BiH3
Which one of the following orders presents the correct sequence of the increasing basic nature of the given oxides?
-
Al2O3 < MgO < Na2O < K2O
-
MgO < K2O < Al2O3 < Na2O
-
Na2O < K2O < MgO < Al2O3
-
K2O < Na2O < Al2O3 < MgO
A.
Al2O3 < MgO < Na2O < K2O
As the metallic character of element attached to oxygen atom increases, the difference between the electronegativity values of element and oxygen increases and thus the basic character of oxides increases and vice-versa. Hence the increasing correct order of basic nature is Al2O3 < MgO < Na2O < K2O.
Which of the following statements regarding sulphur is incorrect?
-
S2 molecule is paramagnetic.
-
The vapour at 200ºC consists mostly of S8 rings.
-
At 600ºC the gas mainly consists of S2 molecules.
-
The oxidation state of sulphur is never less than +4 in its compounds.
D.
The oxidation state of sulphur is never less than +4 in its compounds.
Sulphur exhibit + 2, + 4, + 6 oxidation states but + 4 and + 6 are more common
The structure of IF7 is
-
square pyramid
-
trigonal bipyramid
-
octahedral
-
pentagonal bipyramid
D.
pentagonal bipyramid
The structure is pentagonal bipyramid having sp3d3 hybridisation as given below,
Boron cannot form which one of the following anions?
-
BF63-
-
BH4-
-
B(OH)4-
-
BO2-
A.
BF63-
because of the non-availability of d-orbitals, boron is unable to expand its octet. Therefore, the maximum covalence of boron cannot exceed 4.
29.5 mg of an organic compound containing nitrogen was digested according to Kjeldahl’s method and the evolved ammonia was absorbed in 20 mL of 0.1 M HCl solution. The excess of the acid required 15 mL of 0.1 M NaOH solution for complete neutralization. The percentage of nitrogen in the compound is
-
59.0
-
47.4
-
23.7
-
29.5
C.
23.7
m moles of HCl = 20 × 0.1 = 2
m moles of NaOH = 15 × 0.1 = 1.5
∴ m moles of HCl that consumed NH3 = 0.5
m moles of NH3 = 0.5
milligrams of N in NH3 = 0.5 × 14 = 7 mg
![]()
Which of the following reactions is an example of a redox reaction?
-
XeF4 + O2F2 → XeF6 + O2
-
XeF2 + PF5 → [XeF]+PF6–
-
XeF6 + H2O → XeOF4 + 2HF
-
XeF6 + 2H2O → XeO2F2 + 4HF
A.
XeF4 + O2F2 → XeF6 + O2
In the reaction
![]()
Xenon undergoes oxidation while oxygen undergoes reduction.
The products obtained when chlorine gas reacts with cold and dilute aqueous NaOH are
-
ClO– and ClO3–
-
ClO2- and ClO3-
-
Cl– and ClO–
-
Cl– and ClO2-
C.
Cl– and ClO–
Cl2 + 2OH- (cold ) → Cl- + ClO- +H2O
A liquid was mixed with ethanol and a drop of concentrated H2SO4 was added. A compound with a fruity smell was formed. The liquid was:
-
CH3OH
-
HCHO
-
CH3COCH3
-
CH3COOH
D.
CH3COOH
The fruity smell is evolved due to the formation of ester.
![]()
In which of the following arrangements, the sequence is not strictly according to the property written against it?
-
CO2< SiO2< SnO2< PbO2: increasing oxidising power
-
HF< HCl < HBr < HI : increasing acid strength
-
NH3< PH3< AsH3< SbH3: increasing basic strength
-
B < C < O < N : increasing first ionization enthalpy
C.
NH3< PH3< AsH3< SbH3: increasing basic strength
As the size of the central non-metal increasing appreciably, the basicity of hydride decreases.
NH3< PH3< AsH3< SbH3
Identify the incorrect statement among the following
-
Ozone reacts with SO2 to give SO3
-
Silicon reacts with NaOH(aq) in the presence of air to give Na2SiO3 and H2O
-
Cl2 reacts with excess of NH3 to give N2 and HCl
-
Br2 reacts with hot and strong NaOH solution to give NaBr, NaBrO4 and H2O
D.
Br2 reacts with hot and strong NaOH solution to give NaBr, NaBrO4 and H2O
The molecular shapes of SF4, CF4 and XeF4 are
-
the same with 2,0 and 1 lone pairs of electrons on the central atom, respectively
-
the same with 1, 1 and 1 lone pair of electrons on the central atoms, respectively
-
different with 0, 1 and 2 lone pair of electrons on the central atoms, respectively
-
different with 1, 0 and 2 lone pairs of electron on the central atoms respectively
D.
different with 1, 0 and 2 lone pairs of electron on the central atoms respectively
Heating an aqueous solution of aluminium chloride to dryness will give
-
AlCl3
-
Al2Cl6
-
Al2O3
-
Al(OH)Cl2
C.
Al2O3
Al2Cl66H2O → Al2O3 + + 6HCl + 3H2O↑
In silicon dioxide
-
Each silicon atom is surrounded by four oxygen atoms and each oxygen atom is bonded to two silicon atoms
-
Each silicon atom is surrounded by two oxygen atoms and each oxygen atom is bonded to two silicon atoms
-
Silicon atoms is bonded to two oxygen atoms
-
there are double bonds between silicon and oxygen atoms
A.
Each silicon atom is surrounded by four oxygen atoms and each oxygen atom is bonded to two silicon atoms
Which one the following statement regarding helium is incorrect?
-
It is used to fill gas balloons instead of hydrogen because it is lighter and non – inflammable
-
It is used in gas – cooled nuclear reactors
-
It is used to produce and sustain powerful superconducting reagents
-
It is used as cryogenic agent for carrying out experiments at low temperatures
A.
It is used to fill gas balloons instead of hydrogen because it is lighter and non – inflammable
Helium is not used to produce and sustain powerful superconducting magnets. All others are the uses of helium.
One mole of magnesium nitride on the reaction with an excess of water gives
-
one mole of ammonia
-
two moles of nitric acid
-
Two mole of ammonia
-
one mole of nitric acid
C.
Two mole of ammonia
Mg3N2 (s) + 6H2O (l) → 3Mg(OH)2 + 2NH3 (g)
Aluminium chloride exists as a dimer, Al2Cl6 in the solid state as well as in solution of non-polar solvents such as benzene. When dissolved in water, it gives
-
Al3+ + 3Cl-
-
Al2O3 + 6HCl
-
[Al(OH)6] 3-
-
[Al(H2O)6]3+ + 3Cl-
D.
[Al(H2O)6]3+ + 3Cl-
AlCl3 is covalent but in water, it becomes ionic due to large hydration energy of Al3+
![]()
Match the compounds given in column I with the hybridization and shape given in column II and mark the correct option.
|
Column I |
Column II |
|
A.XeF6 |
1.Distorted octahedral |
|
B.XeO3 |
2.Square planar |
|
C.XeOF4 |
3.Pyramidal |
|
D.XeF4 |
4.Square pyramidal |
-
1 2 4 3ABCD -
4 3 1 2ABCD -
ABCD4 1 2 3
-
1 3 4 2A
B
C
D
D.
|
A |
B |
C |
D |
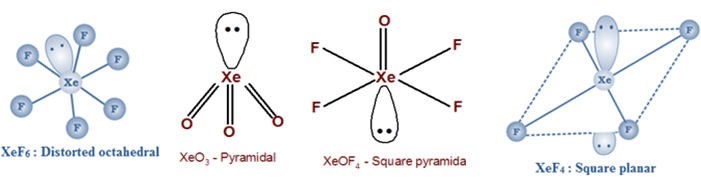
Which is the correct statement for the given acids?
-
Phosphinic acid is a monoprotic acid while phosphonic acid is a diprotic acid.
-
phosphinic acid is a diprotic acid while phosphonic acid is a monoprotic acid
-
Both are triprotic acids
-
Both are diprotic acids
A.
Phosphinic acid is a monoprotic acid while phosphonic acid is a diprotic acid.
Phosphinic acid
Due to the presence of one replaceable proton in phosphinic acid, it is monoprotic acid. and due to the presence of two replaceable proton in phosphinic acid, it is diprotic acid.
The product obtained as a result of a reaction of nitrogen with CaC2 is
-
CaCN
-
CaCN3
-
Ca2CN
-
Ca(CN)2
D.
Ca(CN)2
When calcium carbide reacts with nitrogen under high temperature, it forms calcium cyanamide which is also called nitrolim.
![]()
Which one of the following orders is correct for the bond dissociation enthalpy of halogen molecules?
-
Cl2>Br2>F2>I2
-
Br2>I2>F2>Cl2
-
F2>Cl2>Br2>I2
-
I2 >Br2>Cl2>F2
A.
Cl2>Br2>F2>I2
Bond dissociation energies of halogen family decrease down the group as the size of the atom increases. The bond dissociation energy of fluorine, is, however, lower than those of chlorine and bromine because of interelectronic repulsions present in the small atom of fluorine.
Hence bond energy decreases in the order Cl2 > Br2 > F2 > I2
Among the following, the correct order of acidity is
-
HClO<HClO2<HClO3<HClO4
-
HClO2<HClO<HClO3<HClO4
-
HClO4<HClO<HClO <HClO3
-
HClO3<HClO4<HClO2<HClO
A.
HClO<HClO2<HClO3<HClO4
As the oxidation state of halogen increases the acidity of oxyacid also increases.
HClO: Oxidation state of Cl =+1
HClO2 : Oxidation state of Cl =+3
HClO3 : Oxidation state of Cl = +5
HClO4 : Oxidation state of Cl = +7
Therefore, the correct order of acidity would be
HClO2<HClO<HClO3<HClO4
Which of the following options represents the correct order?
-
O2-> O2>O2+
-
O2-<O2<O2+
-
O2->O2<O2+
-
O2-<O2>O2+
B.
O2-<O2<O2+
Bond order of O2-

Nitogen dioxide and sulphur dioxide have some properties in common. which property is shown by one of these, comounds but not the order?
-
Forms acid -rain
-
Is a reducing -agent
-
Is a soluble in water
-
Is used as a food preservative
D.
Is used as a food preservative
SO2 is used in the manufacture of sodium bisulphate (NaHSO3) which is used as preservatives for jams, jellies and squashes.
The maximum bond angle at nitrogen is present in which of the following?
-
NO2
-
NO2-
-
NO2+
-
NO3-
C.
NO2+
In all of the four molecules NO2- and NO2 have one lone pair thus bond angle is less 120 in the case of NO2- and more than 120 in the case of NO2 but in the NO3- there is no lone pair hence all are bonding pairs leading to an ideal bond angle of 120.
In NO2+, the is no lone pair only bond pair exist thus leading a bond angle is 180.
Acidity of diprotic acids in aqueous solutions increases in the order
-
H2S < H2Se< H2Te
-
H2Se <H2S <H2Te
-
H2Te <H2S < H2Se
-
H2Se < H2Te <H2S
A.
H2S < H2Se< H2Te
Acidic strength of hydrides increases as the size of central atom increases which weakens the M-H bond. Since, the size increases from S to Te thus acidic strength follows the order
H2S <H2Se <H2Te
Maximum deviation from ideal gas expected from
-
H2 (g)
-
N2 (g)
-
CH4 (g)
-
NH3(g)
D.
NH3(g)
Easily liquefiable gases like NH3, SO2 etc. Exhibit maximum deviation from ideal gas as for them Z<<<1.
CH4 also exhibits deviation but it is less as compared to NH3.
Which is the strongest acid in the following?
-
H2SO4
-
HClO3
-
HClO4
-
H2SO3
C.
HClO4
The strength of oxyacids can also be decided with the help of the oxidation number of the central atom. Higher the oxidation number of central atoms, more acidic is the oxyacid.
![]()
Since in HClO3 oxidation number of Cl is highest, so HClO4 is the strongest acid among the given.
Identify the correct order of solubility in aqueous medium
-
CuS> ZnS> Na2S
-
ZnS > Na2S> CuS
-
Na2S> CuS> ZnS
-
Na2S> ZnS> CuS
D.
Na2S> ZnS> CuS
Ionic compounds are more soluble in water or in an aqueous medium.
Ionic character  Size of cation (if cation is same)
Size of cation (if cation is same)
The order os size of cation is
Na+> Zn2+>Cu2+
therefore,
The order of ionic character and hence, of solubility in water is as
XeF2 is isostructural with
-
TeF2
-
ICl-2
-
SbCl3
-
BaCl3
B.
ICl-2
Species having the same number of bond pairs and lone pairs are isostructural (have same structure)
| Species |
lp+bp |
Structure |
|
XeF2 |
4 lp +2bp |
Linear |
|
TeF2 |
2lp +2bp |
Angular or V-shape |
|
ICl2- |
4lp +2bp |
Liner |
|
BaCl2 |
0 lp +2bp |
(linear paper) Cl-Ba-Cl |
Thus, XeF2, is isostructural with ICl2- and BaCl2.
Which one of the following molecules contains no pi -bond?
-
CO2
-
H2O
-
SO2
-
NO2
B.
H2O
All the molecules have O atom with lone pairs, but in H2O the H atom has no vacant orbital for pi-bonding. That's why it does not have any pi- bond.
In all other given molecules, the central atom because of the presence of vacant orbitals is capable to farm Pi- bonds.
Which of the following is a polar molecule?
-
BF3
-
SF4
-
SiF4
-
XeF4
B.
SF4
Symmetrical molecules are generally non-polar although they have polar bonds. This is because bond dipole on one bond is cancelled by that of the other. BF3, SiF4 and XeF4 being symmetrical as non-polar. SF4 is unsymmetrical because of the presence of a lone pair of electrons. Due to which it is a polar molecule.
Which of the following statements is not valid for oxoacids of phosphorus?
-
Orthophosphoric acid is used in the manufacture of triple superphosphate
-
Hypophosphorous acid is a diprotic acid
-
All oxoacids contain tetrahedral four coordinated phosphorus
-
All oxoacids contain at least one P=O unit and one P-OH group
B.
Hypophosphorous acid, H3PO2 has the following structure.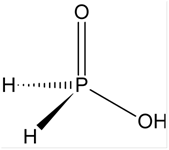
As it contains only one replaceable H-atom (that is attached to O, not with P directly so it is a monoprotic acid.
All other given statements are true.
In Which of the following arrangement, the given sequence is not strictly according to the property indicated against it ?
-
HF < HCl< HBr < HI : increasing acidic strength
-
H2O < H2S < H2Se < H2Te : increasing pKa values.
-
NH3 < PH3 < AsH3 < SbH3 : increasing acidic character
-
CO2 < SiO2 < SnO2 <PbO2 : increasing oxidising power
B.
H2O < H2S < H2Se < H2Te : increasing pKa values.
(a,c) As we move from top to bottom in a group, the acidic strength of hydrides increases. Therefore, order of acidic strength of hydrides of VA and VII A group elements is
NH3 < PH3 < AsH3< SbH3
HF<HCl < HBr <HI
H2O > H2S >H2Se > H2Te
d) On moving from top to bottom, oxidising power of oxides increases. Thus, order of oxidising power of oxides of IVA group elements is
CO2 < SiO2 < SnO2 < PbO2
The stability of +1 oxidation state among Al, Ga, In and TI increases in the sequence
-
Ga<In< Al<Tl
-
Al<Ga<In<Tl
-
Tl<In<Ga<Al
-
In< Tl< Ga<Al
B.
Al<Ga<In<Tl
Al< Ga< In <Tl
This is due to inert pair effect or tendency of ns2 electrons do not participate in bond formation.
This tendency decreases on moving down the group.
The variation of the boiling point of the hydrogen halides in the order HF > HI > HBr > HCl. What explains the higher boiling point of hydrogen fluoride?
-
The electronegativity of fluorine is much higher than for other elements in the group
-
There is strong hydrogen bonding between HF molecules
-
The bond energy of HF molecules is greater than in other hydrogens halides
-
The effect of nuclear shielding is much reduced in fluorine which polarises the HF molecules
B.
There is strong hydrogen bonding between HF molecules
There is a strong hydrogen bonding between HF molecules. Hence, the boiling point is highest for HF.
HF > HI > HBr > HI
Which of the following oxide is amphoteric?
-
SnO2
-
CaO2
-
SiO2
-
CO2
A.
SnO2
SnO2 is an amphoteric oxide because it reacts with acids as well as bases to form corresponding salts.
SnO2 +4HCl → SnCl4 +2H2O
SnO2 + 2 NaOH → Na2SnO3 + H2O
Strong reducing behaviour of H3PO4 is due to
-
the presence of one -OH group and two P-H bonds
-
high electron gain enthalpy of phosphorous
-
the high oxidation state of phosphorus
-
the presence of two -OH groups and one P-H bond
A.
the presence of one -OH group and two P-H bonds
The oxy-acid of phosphorus which contains P- H bond act as a reducing agent or reductant.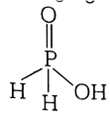
In H3PO2 one -OH group and two P-H bonds are present.
Oxidation states of P in H4P2O5, H4P2O6, H4P2O7, are respectively
-
+3, +5, +4
-
+5, +3, +4
-
+5, +4,+3
-
+3, +4, +5
D.
+3, +4, +5
Oxidation state of H is +1 and that of O is -2
Let the oxidation state of P in the given compound x.
In H4P2O5,
(+1) x 4 +2 X x + (-2) x 5 = 0
4 + 2x -10 = 0
2x = 6
x = 3
In H4P2O6,
(+1) X 4 + 2 X x + (-2) x6 = 0
4 x + 2x-12 = 0
2x = 8
x =4
In H4P2O7,
(+1) x 4 x 2 X x + (-2) x 7 = 0
4 + 2x -14 = 0
2x = 10
x = +5
Thus oxidation states of P in H4P2O5, H4P2O6, H4P2O7, are +3, +4 and +5.
The correct order of increasing bond angles in
-
Cl2O < ClO2 < ClO2-
-
ClO2 <Cl2O< ClO2-
-
Cl2O < ClO2- < ClO2
-
ClO2- < Cl2O < < ClO2
D.
ClO2- < Cl2O < < ClO2
As the number of lone pair of electrons increases, bond angle decreases due to repulsion between lp-lp. Moreover, as the electronegativity of the central atom decreases, bond angle decreases. Hence, the order of bond is
(Cl is less electronegative as compared to O.)
Match (I) substances with List (II) processes employed in the manufacture of the substances and select the correct option.
|
List I |
List II |
||
|
A |
Sulphuric acid |
1 |
Haber’s process |
|
B |
Steel |
2 |
Bessemer’s process |
|
C |
Sodium |
3 |
Lablanc process |
|
D |
Ammonia |
4 |
Contact process |
-
A
B
C
D
1
4 2 3
-
A
B
C
D
1
2
3
4
-
A
B
C
D
4
3 2 1 -
A
B
C
D
4
2 3 1
D.
|
A |
B |
C |
D |
|
4 |
2 | 3 | 1 |
Sulphuric acid is manufactured by contact process, steel is manufactured by Bessemer's process, Lablanc process is used for the production of NaOH while NH3 is obtained by Haber's process.
which of the following oxides is not expected to react with sodium hydroxide?
-
B2O3
-
CaO
-
SiO2
-
BeO
B.
CaO
Generally, acids react with bases and bases (alkalies) react with acids.
sodium hydroxide, NaOH, being a strong alkali, never react with a basic oxide (compound). Among the given options, B2O3 and BeO are amphoteric oxides, SiO2 is a acidic oxide and CaO is a basic oxide. Therefore, NaOH does not react with CaO.
Among the following which is the strongest oxidising agent?
-
F2
-
Br2
-
I2
-
Cl2
A.
F2
An element having higher tendency to get reduced or to accept an electron is a strong oxidising agent.
Fluorine is the most electronegative element because electronegativity decrease on moving down the group. Hence, it gets reduced readily into F- ion and is a strongest oxidising agent.
The stability of +1 oxidation state increases in the sequence
-
Al < Ga <In < Tl
-
Tl < In <Ga< Al
-
In < Tl < Ga < Al
-
Ga < In < Al < Tl
A.
Al < Ga <In < Tl
Stability of lower oxidation states increases on moving down a group due to inert pair effect.
The given elements belongs to third group. These elements mainly exhibit +3 and +1 oxidation state. As we know the stability of lower oxidation state ie, +1 state, increases on moving down a group, the sequence of stability is
Al< Ga< In <Tl
Which one of the following arrangement does not give the correct picture of the trends indicated against it?
-
F2 > Cl2 > Br2 > I2 : Oxidising power
-
F2 > Cl2 > Br2 > I2 : Electron gain enthalpy
-
F2 > Cl2 > Br2 > I2 : Bond dissociation energy
-
F2 > Cl2 > Br2 > I2 : Electronegativity
C.
F2 > Cl2 > Br2 > I2 : Bond dissociation energy
Generally, bond dissociation energies decrease in a group. Bond dissociation energy also decreases with repulsion.
| X-X Bond | F-F | Cl-Cl | Br- Br | I-I |
| Bond length (A) | 1.42 | 1.99 | 2.28 | 2.67 |
| Bond dissociation energy (kcal/mol) | 38 | 57 | 45.5 | 35.6 |
In general, the bond dissociation energy decreases as the bond length increases, but the bond dissociation energy of F2 is less than that of Cl2. It is due to greater interelectronic repulsion between the lone pair of electrons on the two bonded fluorine atoms. Hence, the order of bond dissociation energy is as:
Cl2 > Br2 > F2 > I2
Which one of the following ionic species has the greatest proton affinity to form stable compound?
-
HS-
-
NH2-
-
F-
-
I-
C.
F-
Fluorine is the most electronegative element in the periodic table. So, it has the greatest proton affinity to form stable compounds
Which one of the following anions is present in the chain structure silicates ?
-
Si2O76-
-
(Si2O56-)n
-
(SiO32-)n
-
SiO44-
C.
(SiO32-)n
[SiO32-] and [Si4O11]6- have chain structure of silicates.
For the following:
(i) I- (ii) Cl- (iii) Br-
the increasing order of nucleophilicity would be:
-
I- < Br- < Cl-
-
Cl- < Br- < I-
-
I- < Cl- < Br-
-
Br- < Cl- < I-
A.
I- < Br- < Cl-
Halogens are the most reactive elements. High reactivity is due to their low dissociation energy. Further among halogen reactivity decreases as we move down the group. So, the correct order of nucleophilicity would be
I- < Br- < Cl-
Which one of the following orders correctly represents the increasing acid strengths of the given acids?
-
HOCl < HOClO < HOClO2 < HOClO3
-
HOClO < HOCl < HOClO3 < HOClO2
-
HOClO2 < HOClO3 < HOClO < HOCl
-
HOClO3 < HOClO2 < HOClO < HOCl
D.
HOClO3 < HOClO2 < HOClO < HOCl
The acidic character of oxyacids increases with increases in oxidation number. The acidity of oxyacids of chlorine decreases in the following order.
![]()
So relative acidic character increases.
Which of the following is the most basic oxide?
-
Al2O3
-
Sb2O3
-
Bi2O3
-
SeO2
C.
Bi2O3
In Al2O3, Sb2O3, Bi2O3 and SeO2. Bi2O3 is most basic oxide due to higher reactivity with acid
Bi2O3 + 6HCl → 2BiCl3 + 3H2O
Which one of the following orders is not in accordance with the property stated against it?
-
F2 > Cl2 > Br2 > I2 : Oxidising power
-
HI> HBr> HCl >HF: Acidic property in water
-
F2 > Cl2 > Br2 > I2: Electrongeativity
-
F2 > Cl2 > Br2 > I2 : Bond dissociation energy
D.
F2 > Cl2 > Br2 > I2 : Bond dissociation energy
Incorrect order of bond dissociation energy F2 > Cl2 > Br2 > I2 due to following order of size I > Br> Cl> F.
Ionic mobility of which of the following alkali metal ions is lowest when aqueous solution of their salts are put under an electric field?
-
Na
-
K
-
Rb
-
Li
D.
Li
Li+ being smallest has maximum charge density
∴ Li+ is most heavily hydrated among all alkali metal ions. The effective size of Li+ in aq solution is therefore, largest.
∴ Moves slowest under the electric field.
Which one of the following elements is unable to form ion?
Ga
Al
In
B
D.
B
The element M in the complex-ion has a coordination number of six.
B has only s and p-orbitals and no d – orbitals available, therefore, at the maximum it can show a coordination number of 4. Thus, B cannot form the complex of the type
Which of the following statements is not true for halogens?
All form monobasic oxyacids
All are oxidizing agents
Chlorine has the highest electron-gain enthalpy
All but fluorine show positive oxidation states
D.
All but fluorine show positive oxidation states
Due to high electronegativity and small size, F forms only one oxoacid, HOF known as Fluoric (I) acid the oxidation number of F is +1 in HOF.
Which of the following oxide is most acidic?
As2O3
P2O5
Sb2O3
Bi2O3
B.
P2O5
More be the positive oxidation state of non-metallic oxides, more be its acidic nature.
In which of the following compounds, sulphur show maximum oxidation number?
H2SO4
SO3
H2S2O7
All have same oxidation number for sulphur
D.
All have same oxidation number for sulphur
All have same oxidation number for sulphate i.e + 6
(i) oxidation number of S is
H2SO4 = +2 +x -8 = 0
Therefore, x = +6
(ii) Oxidation number of S is SO3 = x - 6 = 0
Therefore, x = 6
(iii) Oxidation number of S in H2S2O7
= 2 + 2x - 14 = 0
2x = + 12
x = +6
Mock Test Series
Sponsor Area
Sponsor Area