Biology Chapter 9 Biomolecules
Sponsor Area
NCERT Solution For Class 11 Political+science Biology
What are major elements of a cell ?
What is the general formula of carbohydrates ?
Give the names of two acidic aminoacids.
What are the contents of lactose ?
What are carbohydrates ?
Give the names of two vitamin nucleotides.
What are the different types of carbohydrates ?
What are monosaccharides ?
What is glycosidic bond ?
What are heterocyclic aminoacids ?
Sponsor Area
What are disaccharides ?
Name sulphur containing amino acids.
What are polysacharides and state their functions?
Polysaccharides perform various functions -
(1) Source of energy - polysaccharides are broken down to produce energy in the cells.
(2) Storage - The food is stored in the form of starch in plants and in the form of glycogen in animals.
(3)Structural support - Cellulose is the structural material of plants.
(4) Protective covering - Exoskeleton of the arthropods are made of polysaccharide chitin.
What are lipids ?
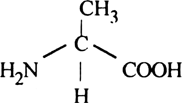
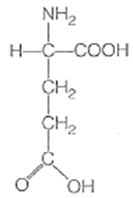
What is amino acid ?
Amino acids are organic compounds containing an amino group and an acidic group as substituents on α-carbon. Thus, they are called α-amino acids. They are substituted methanes. The four valency positions of the carbon are occupied by four different groups which are hydrogen, carboxyl group, amino group and a variable group R group. There are 20 amino acids.
What are rare amino acids. Give an example?
What is the difference between simple and mixed triglycerides ?
Simple triglycerides are are those that have only one kind of fatty acids, whereas the mixed triglycerides are made up of different fatty acids.
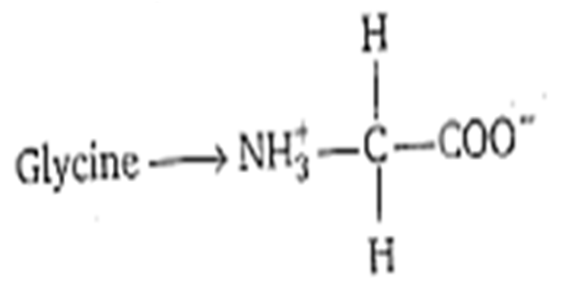
What are amphoteric compounds and Zwitter ions ?
Zwitter ion is a molecule or ion having separate positively and negatively charged groups. For example amino acid which can have positive NH2 and negative COOH group.
Explain composition of triglyceride.

What are essential fatty acids ?
Write a brief note on hydrogenation.
What is ‘magic 20’ ?
Name ten essential amino acids.
What are glycolipids ?
Cholesterol is harmful as it causes blood pressure and heart attack, even then it is considered essential for human body. Why ?
1. Cholesterol is the precursor of many hormones such as progesterone, testosterone, estradiol and Cortisol.
2. Cholesterol provides fluidity to the cell membrane and hence helps in the maintenace of its structure.
What do you mean by ‘vegetable oil rich in polyunsaturates’ ? Why is it recommended by physicians for persons suffering from high blood cholesterol or cardio vascular diseases ?
Physicians recommend these to person's suffering from high blood pressure or cardio vascular diseases, because these lower the blood pressure and thus decreases the risks.
What are phospholipids ? What is their importance in living cells ?
Give three examples of phospholipids.
i. Lecithin.
ii. Cephalins.
iii.Sphingomyelin.
Sponsor Area
Describe the functions of lipids.
1. Lipids act as important storage compounds.
2. They form membranous structures of the cell.
3. Lipids are rich source of energy and the energy produced by 1 gram of fat is more than twice than that produced from 1 gram of glucose.
4. They serve as main parts of cell wall in plants and form exoskeleton of insects.
5. In certain animals such as whale, polar bear etc. they form an insulating layer.
6. They are also used in the synthesis of steroid hormones and bile salts.
7. Fats from cushion like structures below vital organs such as brain, heart, kidneys, eyes etc. and protect them from mechanical shocks.
8. Fats insulate the nerve fibres electrically.
What are the functions of cholesterol?
(1) Cholesterol is a component of plasma membrane.
(2) The cholesterol is a parent compound from which many hormones.
(3) Cholesterol produces bile salts.
(4) It maintains the fluidity of the cell membrane in cold conditions.
What are principle elements ?
What are cutins and suberins ?
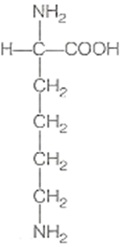
Draw the structure of amino acid.
Amino acid are organic compounds made up of carbon, hydrogen, oxygen and nitrogen. They consist of an amino group and an acidic group attached to the the same carbon called α-carbon. Thus, they are also known as α-amino acids. They are substituted methanes. Four groups occupy the four valency positions. The four groups being an amino group, a hydrogen, A carboxylic group and a variable R group. The R group could be a hydrogen(the amino acid is called glycine), a methyl group (alanine) etc. There are 20 amino acids that occur in proteins.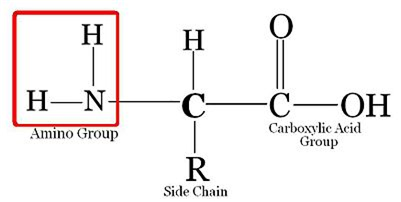
Arctic and Antarctic fishes have unsaturated fatty acids. Why ?
What is lard ?
What are acidic, basic and neutral amino acids ?
The acidic amino acids contain one amino and two carboxyl groups each, e.g. glutamic acid and aspartic acid.
The basic amino acids have two amino groups and one carboyxl group, e.g. lysine and arginine.
The neutral amino acids have one amino group and one carboxyl group e.g. alanine, glycine, valine and phenylalanine.
What is the full form of ATP, ADP and AMP ?
ADP stands for Adenosine Diphosphate.
AMP stands for Adenosine Monophosphate.
How are amino acids bonded together ? Describe how these bonds are formed ?
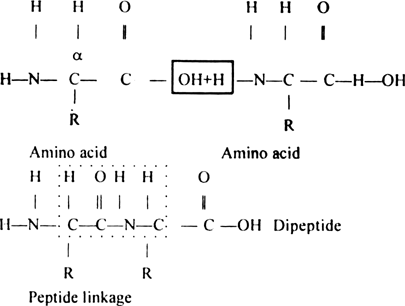
The resulting ![]() linkage is called a peptide linkage and the product is called dipeptide. The C–N bond in the peptide linkage is called a peptide bond.
linkage is called a peptide linkage and the product is called dipeptide. The C–N bond in the peptide linkage is called a peptide bond.
Decsribe any 3 functions of amino acids.
(1) These act as building blocks of proteins and help in their synthesis by polymerisation.
(2) Some of the amino acids are converted into useful biologically active compounds .
(3) Non-protein amino acids serve as different components of antibiotics.
Which is the most abundant protein in the whole biosphere?
What are acidic amino acids ?
What are dextrorotatory amino acids?
What are laevorotatory amino acids ?
Write a note on coenzymes.
Enzymes are dependent upon metal ions for their action. Discuss.
Distinguish between Oligosaccharides and Disaccharides.
|
Oligosaccharides |
Disaccharides |
|
(1) Contain 2 to 10 units of monosaccharides. (2) Slightly soluble in water. (3) Tastelss. (4) Form glycoalyx on plasma membrane. |
(1) Contain 2 units of monosaccharides. (2) Soluble in water. (3) Sweet. (4) Form storage sugars. |
Distinguish between oils and fats.
|
Oils |
Fats |
|
(1) Rich in unsaturated fatty acids. (2) Liquid at ordinary temperature. (3) Contain essential fatty acids.
|
(1) Rich in saturated fatty acids. (2) Solid or semi-solid at ordinary temperature. (3)Do not contain essential fatty acids.
|
Distinguish between unsaturated and saturated fatty acids.
|
Unsaturated fatty acids |
Saturated fatty acids |
|
(1) They have one or two or three double bonds between the carbon atoms of the molecular chain. (2) They have lower melting and boiling points. (3) They cannot be synthesized in the body of any animal and are therefore essential. (4) Are more reactive and do not have a tendency to settle in the body. (5) Generally less harmful and do not cause cardiovascular diseases e.g. oleic acid.
|
(1) They have no double bonds between the atoms of the molecular chain. (2) They have higher melting points and boiling points. (3) They can be synthesized in the animal body and are therefore non-essential. (4) Are more stable and tend to accumulate in the body. (5) Harmfull and can cause cardiovascular diseases e.g. stearic acid.
|
Distinguish between Aldose sugar and Ketose sugar.
|
Aldose sugar |
Ketose Sugar |
|
(1) It has an aldehyde group. (2) First carbon forms a part of aldehyde group. (3) Aldoses are more common in nature e.g. ribose, glucose. |
(1) It has a ketone group. (2) Second carbon forms a part of keto group. (3) Ketose are less common in nature, e.g. ribulose, fructose. |
Distinguish between reducing and non-reducing sugars.
|
Reducing Sugars |
Non-reducing sugars |
|
(1) Give bright red-precipitate with Fehling’s or Benedict’s solutions. (3) Cannot be hydrolysed. (4) All monosaccharides and some disaccharides like maltose and lactose are reducing sugars. |
(1) Do not give bright red-precipitate with Fehling’s or Benedict’s solutions. (2) Do not have free aldehyde or ketone group to reduce Cu++ to Cu+. (3) On hydrolysis give rise to reducing sugars. (4) Sucrose is non-reducing sugar. |
Distinguish between Glycosidic bond and Peptide bond. Give one similarity
|
Glycosidic bond |
Peptide bond |
|
(1) Join monosaccharide units. (2) Forms by the reaction between aldehyde or ketone group and alcohol groups. (3) Links two carbon atoms through an oxygen atom.
|
(1) Join amino acid units. (2) Forms by reaction between amino acids and carboxyl groups. (3) Links carbon and nitrogen atoms. |
Both glycosidic and peptide bonds involve the elimination of water molecule that is both are dehydration processes.
Distinguish between pyranose and furanose rings.
|
Pyranose ring |
Furanose ring |
|
(1) It is hexagonal. (2) It has five carbon atoms in the ring and one oxygen atom. e.g. glucose, galactose. |
(1) It is pentagonal. (2) It has four carbon atoms and one oxygen atom in the ring. e.g. fructose. |
Distinguish between Purines and Pyrimidines.
|
Purines |
Pyrmidines |
|
(1) Its molecules is double ringed. (2) It is a nine membered ring. (3) Its molecule is larger. (5) For eg. Adenine and guanine. |
(1) Its molecule is single ringed. (2) It is six membered ring. (3) lIts molecule is smaller. (4) For e.g Thymine and cytosine. |
Write any three characteristic features of monosaccharides ?
(1) They are soluble in water.
(2) They have sweet taste and char on heating.
(3) Most of them are reducing in nature.
Describe nucleotides.
(1) Nitrogen containing ring organic compound or heterocyclic compound. It can be adenine, guanine, cytosine, thymine (in DNA), uracil (in RNA).
(2) A pentose sugar. The pentose sugar is ribose in case of RNA and 2' deoxyribose in case of DNA.
(3) A molecule of phosphoric acid.
What are macromolecules ? Give examples.
Give two examples of proteins which transport materials.
(1) Haemoglobin. It transports oxygen to all the parts of the body.
(2) P-protein. This transports organic materials through, phloem in plants.
What is meant by a primary structure of a nucleic acid ?
What are the basic units of :
(1) DNA (2) RNA ?
(1) Deoxyribonucleotide is the basic unit of DNA.
(2) Ribonucleotide is the basic unit of RNA.
Name the three types of RNA.
What is the role of contractile proteins in the body of animals ?
Where are nucleoproteins present in the cell ?
Where is glycogen stored in the body of mammals ?
Sponsor Area
Give two examples of storage carbohydrates.
(1) Starch in plants.
(2) Glycogen in animals.
What are the two types of polysaccharides classified on the basis of their function ?
(1) Structural polysaccharides which provide structural support. e.g. cellulose.
(2) Food storage polysaccharides which are used as storage forms e.g. starch
Name a polysaccharide made up of more than one type of monosaccharides.
Name four biologically most important macromolecules.
(1) polysaccharides (2) proteins (3) nucleic acids (4) lipids.
Suggest the name of three polysaccharides.
What are the advantages of storing carbohydrates in the form of polysaccharides?
(1) Polysaccharides are less bulky than monosaccharides as these are formed by dehydration synthesis (removal of water).
(2) These are easy to store; because they are insoluble in water, therefore, osmotically inactive.
(3) These can easily be broken down by enzymes for release of energy.
What is chitin ?
Chitin is a complex polysaccharide which has a branched, linear chain formed by nitrogen containing monosaccharide N-acetyl glucosamine. It is a homoplymer. It forms the exoskeletons of arthropods and cell wall of bacteria and fungi.
What are the pyrimidines of DNA strands?
Discuss the functions of structural polysaccharides in plants.
(1) They give shape to the cell.
(2) They provide protection.
(3) They prevent bursting of cells in water.
(4) They form fibres for mechanical support.
(5) They are a major contituent of the cell wall.
Give the functions of structural polysaccharides in animals.
(1) Give shape to the body
(2) Provide protection
(3) Prevent dehydration
(4) Check the entry of pathogens.
In which organisms the RNA is genetic material ?
What are the similarities among carbohydrates, proteins and nucleic acids ?
(1) All are macromolecules made up of many repeating units.
(2) All have molecular weight ranging from 18 to 800 Da.
(3) All are found in the acid insoluble fraction.
(4) Bonds between subunits are formed by dehydration synthesis (elimination of water).
Describe the functions of polysaccharides in living organs.
1. Reserve food-materials. Some of the polysaccharides reserve food in animal and plant cells. The reserve food material in animals is glycogen whereas starch is of the plants.
2. Fuel. They are used as fuel. These polysaccharides are converted into monosaccharides and are oxidized for the release of energy.
3. Structural component. Some of the polysaccharides are structural component of the cells. For example some polysaccharides are involved in the formation of biomembranes. Cellulose and chitin are the main structural components.
4. Protective coat. Cell membrane made up of polysaccharides protects the cell.
Describe primary structure of protein.
What is α-helix ?
Functions of a helix. It provides flexibility, elasticity and stability to polypeptide chain.
What is the  pleated sheet structure of a protein.
pleated sheet structure of a protein.

β-pleated sheets of polypeptides
What is meant by tertiary structure of proteins ?
What are quarternary proteins ?
All proteins are made up of same amino acids, explain how human protein may be different from those of a dog.
How are complementary base pairs joined in a DNA double helix ?
(2) A always pairs with T, G always pair with C.
(3) A-T pair has two hydrogen bonds, whereas G-C pair has three hydorgen bonds.
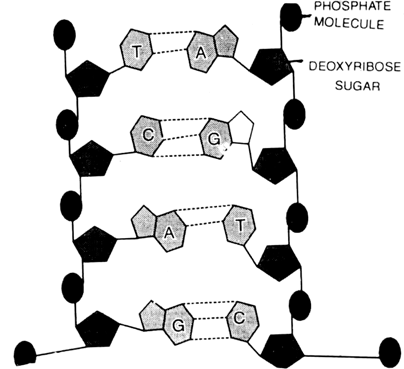
Complementary base pairing of DNA
Differentiate between A, B, C, D and Z-DNAs in tabulated form.
|
Characters |
A-DNA |
B-DNA |
Z-DNA |
|
1. Base pair per turn of the helix. 2. Tilt of base pairs 3. Axial rise (h) 4. Helical diameter (A) 5. Rotation of the double helix DNA. |
11 20.2 Å 2.56 Å 23 Å right handed |
10 6.3 Å 3.37 Å 20 Å right handed |
12 7 Å 3.7Å 18Å left handed |
Describe the types of proteins on the basis of composition.
There are three types on the basis of composition :
1. Simple proteins 2. Conjugated proteins 3. Derived proteins.
1. Simple proteins. Simple proteins consist of only amino acids or their derivatives.
2. Conjugated proteins. These consist of simple proteins in combination with some non-protein component. The non-protein groups are called prosthetic groups.
3. Derived proteins. These are derived from pre-existing proteins either by partial hydrolysis or by coagulation.
Describe the functions of proteins.
1. As enzymes. Many proteins function as enzymes to catalyse specific chemical reactions. For eg trypsin, pepsin etc.
2. As carriers. Some proteins act as carriers which bind and transport specific molecules across a membrane or in a body fluid. GLUT-4 enables glucose transport into cells.
3. Nutrient and storage proteins. Seeds of many plants store nutrient proteins required for the growth of embryonic plant as in wheat, corn. Ovalbumin is the protein of egg and casein is the milk protein.
4. Contractile or motile proteins. Proteins actin and myosin are filamentous proteins found in the contractile system of skeletal muscles which help in the movement.
5. Structural proteins. Some fibrous proteins form supporting filaments. Tendons are formed of collagen proteins and ligaments of elastin; keratin is found in hair, nails and feathers.
6. Defence proteins. Immunoglobins or antibodies form immune system of the body. These recognize bacteria, viruses or foreign proteins.
7. Regulatory proteins. Certain hormones or antibodies are globular proteins (such as insulin). They regulate cellular or physiological functions.
8. Receptor proteins. Receoptors proteins help in the reception of smell, taste. hormone etc.
9. Blood clotting. Proteins like fibrinogens help in blood clotting.
Describe Watson and Crick model of DNA.
According to Watson and Crick model of DNA :
1. The Double Helix consists of two polynucleotide chains, which are coiled like a rope in helical (spiral) fashion.
2. The two strands of polynucleotides are run in the opposite direction and are antiparallel.
3. The backbone is formed by the sugar-phosphate-sugar chain.
4. The nitrogen bases lie perpendicular to this
backbone but face inside.
5. A always pairs with T and G pairs with C. A and T are joined by two hydrogen bonds while the G and C pair have three bonds.
6. A base pair represents each step of ascent of the spiral. At each step the strand turns 36.
7. There are ten base pairs in a complete turn which has ten steps.
8. The pitch is 34Å. the rise per base pair is 3.4Å.
The Watson-Crick model of DNA.
What is RNA ? Describe its different forms.
What role temperature plays in food preservation ?
Describe nomenclature of enzymes.
The six classes of enezymes are -:
i. Oxidoreductases/dehydrogeneases : These are enzymes which catalyse oxidoredcuction reactions.
ii. Transferases: Enzymes catalysing transfer of group between a pair of substrates.
iii. Hydrolases Enzymes catalysing hydrolysis reactions.
iv. Lyases - Enzymes that catalyse removal of groups from substrates by methods other than hydrolysis, leaving double bonds.
v. Isomerases - Enzymes which carry out interconversion reactions.
vi. Ligases : Enzymes catalysing the linking of two compounds.
Write a note on cellulose.
Give two similarities between inorganic catalyst and enzymes.
(1) Both are required in small quantities as compared to the substrate.
(2) Both do not change the equilibrium of a reversible reaction.
Differentiate between RNA and DNA.
|
RNA |
DNA |
|
1. RNA is genetic material only in some viruses.
|
1. It is genetic material of all organisms having cellular structure. 2. It has deoxyribose sugar in it. |
Describe the importance of enzymes.
what do you mean by the term active site of an enzyme.
Find and write down structure of 10 interesting small molecular weight biomolecules. Find if there is any industry which manufactures the compounds by isolation. Find out who are the buyers.
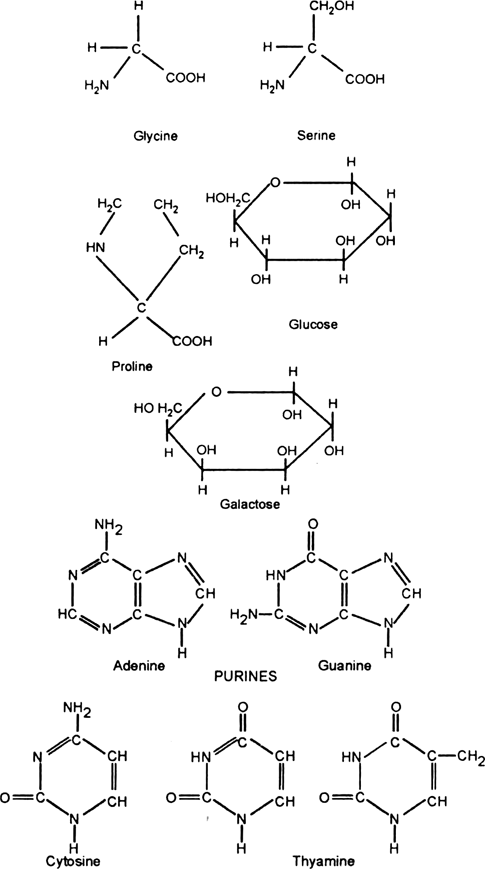
PYRIMIDINES
Purines and pyrimidines are manufactured by isolation. They are used by various laboratories, hospitals and research centres.
What are isoenzymes ?
For example of isoenzymes of lactic dehydrogenase (LDH).
Sponsor Area
What are macromolecules ? Give examples.
Distinguish between Isomerases and transferases.
|
Isomerases |
Transferases |
|
(1) These enzymes catalyse interconversion of isomers
(3) For eg Glucose isomerase which converts glucose to fructose. |
(1) These enzymes catalyse the the transfer of a group between a pair of subtrates. (2) Does not involve re-arrangemnet. |
Illustrate a glycosidic, peptide and a phosphodiester bond.

(2) Peptide bond: Amino acids in a polypeptide or protein are linked by a peptide bond, which is formed when the carboxyl group ie. COOH of one amino acid reacts with the amino (–NH2) group of the next amino acid with the elimination of a water molecule . 
(3) Phosphodiester bond : In a nucleic acid the phosphate group and sugar are joined together by phosphodiester bonds. An bonds is formed between phosphate and pentose sugar molecule in a nucleotide. The bond is called phosphodiester bond because there is an ester bond on either side. 
Proteins have primary structure. If you are given a method to know which amino acid is at either of the two termini (ends) of a protein, can you connect this information to purify or homogeneity of a protein?
Distinguish between Apoenzymes and coenzymes.
|
Apoenzyme |
Coenzyme |
|
(i) It is the protein part of an enzyme. (ii) It is composed of proteins only. (iii) It is thermolabile, affected by heat. (iv) It has catalytic function. |
(i) It is the non-protein part of an enzyme. (ii) Organic compound other than protein are present. (iii) Usually heat resistant. (iv) Has activating effect on enzyme. |
Distinguish between Lyase and ligase..
|
Lyase |
Ligase |
|
(i) Catalyses removal of groups from substrates. (ii) ATP is not consumed |
(i) Catalyses formation of covalent bonds and joining together of two compounds. . (ii) Energy is consumed. |
Distinguish between prosthetic group and cofactors.
|
Prosthetic group |
Co-factors |
|
1. It is a non protein which helps in functioning of enzymes. 2. It may be co-enzyme or co-factor e.g. NAD, FAD (Coenzyme). Fe+2, Cs cofactor. |
1. It is a metal ion which helps in functioning of enzymes 2. It cannot be coenzyme e.g. Zn+2 helps in the functioning of carbonic anhydrase. |
Distinguish between hormones and enzymes.
| Hormones | Enzymes |
| 1. Hormones may be amino acid derivatives, peptides, proteins or steroids in nature. | 1. All enzymes are complex proteins. |
| 2. They have low molecular weight and often readily diffuse through cell membranes. | 2. They have very high molecular weight and are not diffusible. |
| 3. They are secreted by cells at one site and pass via blood to another site to act. | 3. They are secreted by cells and may act in the cells themselves or pass via ducts to act in some cavity in the body. |
| 4. They are used up in their regulatory action. | 4. They remain unaffected in the reaction they catalyze. |
| 5. They may act slowly or quickly. | 5. They act quickly. |
Differentiate between inorganic catalysts and enzymes.
|
Inorganic catalysts |
Enzymes |
|
1. These are simple mineral ions or small molecules. 2. These can catalyse diverse reaction. 3. They are not regulated by any regulator molecules. 4. They are less sensitive to temperature and pH. 5. They have low molecular weight. |
1. These are proteins with complex three dimensional structures. 2. They catalyse only specific reactions of a single or only a few substrates. 3. They can be regulated by specific molecules which can change conformation and hence activity. 4. They are more sensitive to change in temperature and pH. 5. They have high molecular weight. |
Can you describe what happens when milk is converted into curd or yoghurt from your understanding of proteins.
Differentiate between Hydrolase and lyase.
|
Hydrolases |
Lyases |
|
(i) These enzymes catalyse splitting of complex molecules into simple ones by addition of water (hydrolysis). AB + HOH → AH + BOH e.g. Amylase catalyses hydrolysis of starch to sugars. |
(i) These enzymes catalyse splitting of complex molecules into simpler products without water. AB → A + B e.g. Histidine decarboxylase catalyse conversion of histidine to histamine and CO2. |
Write notes on competitive inhibition.
For example - ihibition of substrate dehydrogenase by malonate. In this malonate resembles the substrate succinic acid in structure.
What are gums made of ? Is Fevicol different ?
Can you attempt building models of biomolecules using commercially available atomic models (Ball and Stick Models) ?
For example the ball and stick model for glucose molecule is shown below.

How the enzymes speed up the rate of reaction ?
Enzymes can accelerate reactions by:
i. stabilizing the transition state (substrate+enzyme complex) of a reaction.
ii. providing an alternative reaction pathway for a biological reaction.
Attempt titrating an amino acid against a weak base and discover the number of dissociating (ionizable) functional groups in the amino acid.
Describe various characteristics of enzymes.
1. Molecular weight. Enzymes are substances of high molecular weight.
2. Heat sensitivity. Enzymes are inactivated or destroyed by heat. Inactivation takes place even at very low temperatures.
3. Catalytic properties. The enzymes speed up the reaction and the enzyme is unchanged after the reaction. Thus they act as catalyst.
4. Turn over number. It is the number of substrate molecules which are acted upon by one molecule of enzymes just in one minute. Enzymes have high turn over number.
5. Specificity of enzymes. Enzymes are highly specific in nature i.e. a particular enzyme can catalyse only a particular type of reaction.
Find out and make a list of proteins used as a therapeutic agents. Find other application of proteins (e.g. cosmetic etc)
Proteins that are used as therapeutic agents are as follows:
1. Thrombin and fibrinogen – They help in blood clotting.
2. Insulin – It is used in diabetes as it helps in maintaining blood glucose level in the body.
3. Renin – It helps in osmoregulation.
4. Lactoferrin - It is used as an anitimicrobial.
5. Trypsin - It is used in pharmaceutical.
Proteins are also commonly used in the manufacture of cosmetics, biological buffers, enzymes etc.
Describe. lock and key hypothesis.
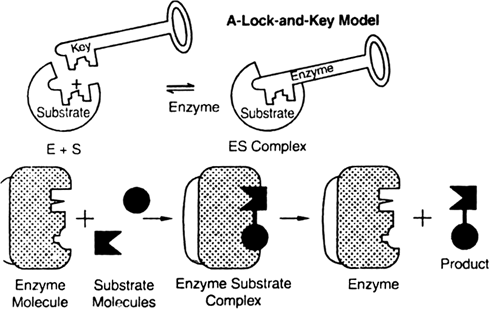
Lock and Key Hypothesis of Enzyme Action Complex
Find out a qualitative test for proteins, fats, oils, amino acids and test any fruit juice, saliva, sweat and urine for them.
(a) For proteins - Biuret's test. If Biuret’s reagent is added to protein,and the colour of the reagent changes from light blue to purple it shows the presence of protein.
(b) For fats or oils - Grease and solubility of the test.
(c) For amino acids - Ninhydrin test. If Ninhydrin reagent is added to the solution, then the colourless solution changes to pink, blue, or purple, it shoes the presence of amino acids.
On testing the fruit juice, saliva, sweat and urine for the molecules we get the following result.
| Substance | Biuret's test for protein | Grease or solubulity test for fats and oil | Ninhydrin test for amino acids. |
| 1. Fruit juice | Gives positive result showing the presence of proteins. | Negative result showing absence of fats and oils. | Gives positive result for the presence of amino acids. |
| 2. Saliva | Shows presence of proteins | Absence of fats and oils | Shows presence of amino acids. |
| 3. Sweat | Absence of proteins | Presence of fats or opils | Absence of amino acids |
| 4. Urine | Presence of proteins. | Not present | Presence of amino-acids. |
Describe. lock and key hypothesis.
Lock and key hypothesis. The hypothesis was put forwarded by Fischer (1894).
1. According to this hypothesis the enzyme and its substrate have a complementary shape like that of a lock and key.
2. The specific substrate molecules are bound to specific region of the enzyme molecule called active site. Just as particular lock can be opened by a particular key specially designed to open it, similarly enzymes have specific sites where a particular substrate can only be attached.
Find out how much cellulose is made by all the plants in the biosphere and compare it with how much of paper is manufactured by man and hence what is the consumption of plant material by man annually. What is the loss of vegetation.
Describe induced fit hypothesis.
Define hormone.
Name the various classes of enzymes.
1. Oxidoreductases.
2. Transferases.
3. Hydrolases.
4. Lyases.
5. Isomerases.
6. Ligase or synthetase.
Describe the various factors which affect enzymatic reactions.
1. Temperature. Enzymes work at optimum temperature. Very high or very low temperatures decreases the activity of enzymes.
Effect of temperature on enzymatic reaction
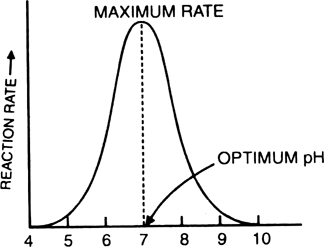
2. Effect of pH value on enzyme reaction - The enzymes are very sensitive towards any changes in the pH of the medium. A particular enzyme can react only at specific pH.
3. Effect of substrate (S) concentration on velocity of reaction- Enzyme activity increases with an increase in concentration of the substrate to a maximum and then it falls off.
4. Binding of specific chemicals - Activators enhance the activity of the enzymes whereas inhibitors decrease the activity of enzymes.
Explain Prothetic group , holoenzyme and enzyme.
The enzymes are proteinous molecules. They are made up of carbon, hydrogen, oxygen and nitrogen. The enzyme molecules may function in the presence of non-proteinous part called prosthetic group. The prosthetic group may be coenzyme or cofactor. Such an enzyme which functions along with co-factor is called apoenzyme. The apoenzyme and prosthetic group are collectively called holoenzyme.
Holoenzyme = Apoenzyme + Prosthetic group.
What are macromolecules? Give examples.
The molecules found in the acid-soluble fraction having a molecular weight in the range of 10,000 daltons or above are known as the macromolecules of the cell.
These are four different types of macromolecules found in the cell . They are:
i. Proteins
ii. Lipids
iii. Nucleic Acids
iv. Polysaccharides.
Describe the important properties of enzymes.
The important properties of enzymes are:
1. All enzymes are proteins. They are complex macromolecules with high molecular weight.
2. They catalyse biochemical reactions in a cell. They help in the breakdown of large molecules into smaller molecules or bring together two smaller molecules to form a larger molecule.
3. They catalyse reactions at a high rate with the help of the active site. They lower down the activation energy and thus the reaction can proceed with ease.
4. Enzymes do not initiate a reaction but accelerate it.
5. They are not used up in the reaction.
6. Enzymes affect the rate and not the direction.
7. Most of the enzymes have a high turnover number that is the number of molecules of a substance that is acted upon by an enzyme per minute.
8. Enzymes are specific in action.
9. Enzymatic activity is affected by changes in temperature, pH and concentration of the substrate.
Which one of the following statements is wrong?
-
cellulose is a polysaccharide
-
Uracil is a pyrimidine
-
Glycine is a sulphur containing amino acid
-
Sucrose is a disaccharide
C.
Glycine is a sulphur containing amino acid
Glycine does not contain sulphur
The amino acid, trytophan is the precussor for the synthesis of
-
thyroxine and tri-iodothryonine
-
estrogen and progesterone
-
cortisol and cortisone
-
melatonin and serotonin
D.
melatonin and serotonin
Melatonin and serotonin are derivatives of tryptophan amino acid.
A typical fat molecule is made up of
-
One glycerol and three fatty acid molecules
-
One glycerol and one fatty acid moleule
-
Thre glycerol and three fatty acid molecules
-
Three glycerol molecules and one fatty acid molecule
A.
One glycerol and three fatty acid molecules
A typical fat molecule is a triglyceride formed by the esterification of one glycerol and three fatty acid molecules.
Transition state structure of the substrate formed during an enzymatic reaction is
-
transient but stable
-
permanent but unstable
-
transient and unstable
-
permanent and stable
C.
transient and unstable
The substrate binds to the enzyme at its active site forming an enzyme-substrate complex. This complex formation is a transient and unstable phenomenon. very soon, the product is released from the active site. It is the fact that all other intermediate structural states are unstable. Stability is related to energy status of the molecule or the structure.
Which of the metabolites is common to respiration mediated breakdown of fats, carbohydrates and proteins?
-
Glucose-6-phosphate
-
Fructose 1, 6-biphosphate
-
Pyruvic acid
-
Acetyl Co-A
D.
Acetyl Co-A
Acetyl Co-A is common to respiration mediated breakdown of fats, carbohydrates and proteins Glucose and fructose are phosphorylated to give rise to glucose-6- phosphate by the activity to the enzyme hexokinase.
Glucose -6- phosphate then converts into fructose 6 phosphate and further to fructose 1-6- biphosphate. Pyruvic acid is the end product of glycolysis.
Macromolecule chitin is
-
Nitrogen containing polysaccharide
-
phosphorus-containing polysaccharide
-
sulphur containing polysaccharide
-
simple polysaccharide
A.
Nitrogen containing polysaccharide
Macromolecule chitin is a complex polysaccharide containing amino sugars and chemically modified sugars (e.g., glucosamine, N -acetylgalactosamine, etc.) Polysaccharides are long carbohydrates molecules of monosaccharide units joined together by glycosidic bonds. They have general fromula (Cx(H2O)y.
The essential chemical components of many coenzymes are
-
proteins
-
nuclei acids
-
carbohydrates
-
vitamins
D.
vitamins
Essential chemical components of many coenzymes are vitamins, e.g., coenzyme Nicotinamide Adeninne Dinucleotide (NAD) acid NADP contain the vitamin niacin Proteins, nucleic acids and carbohydrates are not enzymatic biomolecules.
Which of the following biomolecules does have a phosphodiester bond?
-
Fatty acids in a diglyceride
-
Monosaccharides in a polysaccharide
-
Amino acids in a polypeptide
-
Nucleic acids in a nucleotide
D.
Nucleic acids in a nucleotide
Phosphodiester bond is in responsible for linking nucleotides in nucleic acid (DNA and RNA).
Select the option which is not correct with respect to enzyme action.
-
Substrate binds with enzyme as its active site
-
Addition of lot of succinate does not reverse the inhibition of succinic dehydrogenase by malonate
-
A non- competitive inhibitor binds the enzyme at asite distinct from that which binds as the substrate
-
Malonate is a competitive inhibitor of succinic dehydrogenase
B.
Addition of lot of succinate does not reverse the inhibition of succinic dehydrogenase by malonate
Inhibition of succinic dehyrogenase by malonate is an example of competitive in inhibition.
Cometitive inhibition occurs when enzyme and inhibitor both have more or less similar structure are present in higher concentration.
Thus, both enzyme and inhibitor for competer for the acitve site of enzyme resulting to the decrease iin the enzymatic actively.
Which one of the following is a non-reducing carbohydrate?
-
Maltose
-
Sucrose
-
Lactose
-
Ribose 5 - phosphate
B.
Sucrose
Sucrose is a disaccharide of glucose 8 fructose. It is a non- reducing sugar as it do not contain any free anomeric c arbon atom. Maltose is a disaccharide of 2 glucose units. Its first glucose residue cannot undergo oxidation, whereas, second residue can undergo oxidation because it has a reactive free anomeric carbon atom. Hence, it is a reducing sugar.
Lactose and ribose - 5- phosphate are also reducing in nature due to the presence of a free ketonic or aldehyde group.
The enzyme recombinase is required at which stage of meiosis
-
Pachytene
-
Zygotene
-
Diplotene
-
Diakinesis
D.
Diakinesis
Crossing over is an enzymatic process occurring during th pachytene stage of prophase-1. The enzyme involved in this process is called recombinase which acids in the recombination of genes between homologous chromosomes.
During zygotene stage, homologous chromosomes pair up by process called synapsis and form a complex bivalent structure.
Diplotene is marked by the dissolutino of synaptonemal complex and chaisma formation while diakinesis is marked by terminalisation of chaismata (i.e., chaismata shifts towards periphery of chromosome).
Which one is the most abundant protein in the animal world?
-
Trypsin
-
Haemoglobin
-
Collagen
-
Insulin
C.
Collagen
Collagen is the most abundant protein (structural protein) in the animal world while Ribulose Bisphosphate Carboxylase Oxygenase (RUBISCO) is the most abundant protein in the whole of the plant world.
Which one out of A – D given below correctly represents the structural formula of the basic amino acid?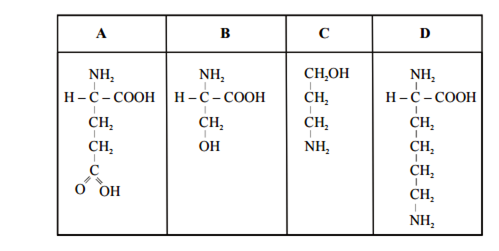
-
C
-
D
-
A
-
B
B.
D
Strucutres D represent basic amino acid lysine whereas structure A represents glutamic amino acid (acid group) and structure B represents alcoholic amino acid serine.
For its activity, carboxypeptidase requires
-
Zinc
-
Iron
-
Niacin
-
Copper
A.
Zinc
Carboxypeptidase is synthesized and secreted by the exocrine cells of pancreas. Zn2+ is required for the activity of carboxypeptidase, carbonic anhydrase and alcohol dehydrogenase.
Which one of the following biomolecules is correctly characterised?
-
Lecithin – A phosphorylated glyceride found in cell membrane
-
Palmitic acid – An unsaturated fatty acid with 18 carbon atoms
-
Adenylic acid – adenosine with a glucose phosphate molecule
-
Alanine amino acid – Contains an amino group and an acidic group anywhere in the molecule
A.
Lecithin – A phosphorylated glyceride found in cell membrane
Alanine is a neutral amino acid with non-cyclic hydrocarbon chain, one amino group and one carboxyl group. Palmitic acid is saturated fatty acid adenylic acid has pentose sugar in it not glucose.
Which one of the following statements in incorrect?
-
A competitive inhibitor reacts reversibly with the enzyme to form an enzymeinhibitor complex
-
In competitive inhibition, the inhibitor molecule is not chemically changed by the enzyme.
-
The competitive inhibitor does not affect the rate of breakdown of the enzymesubstrate complex.
-
The presence of the competitive inhibitor decreases the Km of the enzyme for the substrate.
D.
The presence of the competitive inhibitor decreases the Km of the enzyme for the substrate.
Competitive inhibitor resembles closely with the substrate and competes for the active binding site of the enzyme.
The presence of competitive inhibitor decreases the affinity of the substrate to binding site of the enzyme which results in increase of Michaelis-Menten constant (km).
Which of the following enzymes carries out initial step in the digestion of milk in humans?
-
Rennin
-
Lipase
-
Trypsin
-
Pepsin
D.
Pepsin
In human milk protein digesting enzyme in stomach is pepsin.In calves it is renin.It is alos present in small amounts in human infants but not adults. Pepsin acts on water soluble 'caseinogen (milk protein) to form soluble 'casein'. This combines with calcium salts to form insoulble calcium paracaseinate, which gets readily digested enzymatically.
The curve given below shows enzymatic activity with relation to three conditions (pH, temperature and substrate concentration)
What do the two axises (X and Y) represent?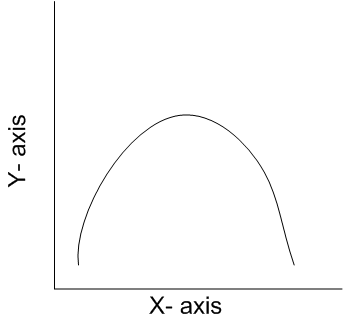
-
X –axis
Y-axis
Temperature
Enzyme activity
-
X –axis
Y-axis
Substrate concentration
enzymatic activity
-
X –axis
Y-axis
enzymatic activity
temperature -
X –axis
Y-axis
enzymatic activity
pH
A.
|
X –axis |
Y-axis |
|
Temperature |
Enzyme activity |
X -axis represents temperature and Y-axis represent enzyme activity. All enzyme act at an optimum temperature, above and below this temperature, the enzyme acitivity declines.
Three of the following statements about enzymes are correct and one is wrong. which one is wrong?
-
Enymes require optimum pH for maximal activity
-
Enzymes are denatured at high temperature but in certain exceptional organisms they are effective even at temperatures 80o-90o C
-
Enzymes are highly specific
-
Most enzymes are proteins but some are lipids
D.
Most enzymes are proteins but some are lipids
Enzymes show pH sensitivity and require an optimum pH for maximal acitivity.
Enzymes are thermolabile (heat sensitive) and denatured at high temperatures. However, extremophiles (thermophilic bacteria) are exceptions. Most of the enzymes are highly specific in their action. Most enzymes are proteins but exceptionally some are RNA enzymes, eg, ribozymes.
The 3' 5' phosphodiester linkages inside a polynucleotide chain serve to join
-
one DNA strand with the other DNA strand
-
one nucleoside with another nucleoside
-
one nucleotide with another nucleotide
-
one nitrogenous base with pentose sugar
C.
one nucleotide with another nucleotide
In a polynucleotide chain 3'-5' phosphodiester bond is formed between carbon 3 of one nucleotide and carbon 5 of the other nucleotide, ie it serves to join one nucleotide with another nucleotide in a polynucleotide chain.
The figure given below shows the conversion of a substrate to product by an enzyme. In which one of the four option (a-d), the components of reaction labelled as A, B, C and D are identified correctly?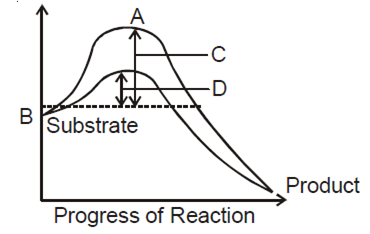
-
A
B
C
D
Potential energy
Transition state
Activation energy with enzyme
Activation energy without enzyme
-
A
B
C
D
Transition energy
Potential energy
Activation energy with enzyme
Activation energy with out enzyme
-
A
B
C
D
Potential energy
Transition energy
Activation energy with out enzyme
Activation energy with enzyme
-
A
B
C
D
Activation energy with enzyme
Transition energy
Activation energy with out enzyme
Potential energy
B.
|
A |
B |
C |
D |
|
Transition energy |
Potential energy |
Activation energy with enzyme |
Activation energy with out enzyme |
The amount of energy required to raise the energy of molecules at which chemical reaction can occur is called activation energy. Thus, acitvatgion energy is actually the energy required to form the transition state. Enzymes dramatically reduce the activation energy of a reaction, so that most molecules can easily get over the activation energy barrier and quickly turn into product. Simply we can say that activation energy of an enzyme catalysed reaction is lower than that of an uncatalysed reaction.
The genetic defect-Adenosine Deaminase (ADA) deficiency may be cured permanently by
-
periodic infusion of genetically engineered lymphocytes having functional ADA cDNA
-
administering denosine deaminase activators
-
introducing bone marrow cells producing ADA into cells at early embryonic stages
-
enzyme replacement therapy
A.
periodic infusion of genetically engineered lymphocytes having functional ADA cDNA
severe combined immunodeficiency (SCID) caused by adenosine deaminase deficiency (AIDA) is the first genetic disorder to be treated with gene therapy. T-cell-directed gene transfer was useful in the treatment of ADA-SCID, whereas the retroviral-mediated gene transfer to haematopoietic stem cells was insufficient for the achievement of clinical benefits.
Carbohydrates are commonly found as starch in plant storage organs. which of the following five properties of starch (A-E) make it useful as a storage material?
A) Easily translocated
B) Chemically non-reactive
C) Easily digested by animals
D) Osmotically inactive
E) Synthesized during photosynthesis
The useful properties are
-
(B) and (C)
-
(B) and (D)
-
(A), (C) and (E)
-
(A) and (E)
C.
(A), (C) and (E)
Starch is a high molecular weight polymer of D-glucose in the alpha 1→4 linkage. It is synthesised in chloroplasts as it is one of the stable end product of photosynthesis. It is most abundant and common storage polysaccharide in plants hence, a most staple food for man and herbivores. It is a mixture of two types of glucose homopolysaccharide viz, amylose and amylopectin. During day time the starch synthesis in chloroplast is coordinated with sucrose synthesis in the cytosol. Typically about 90% of total solute carried in phloem is the carbohydrate sucrose. a disaccharide. This is relatively inactive and highly soluble sugar playing little direct role in metabolism and so making an ideal transport sugar.
One gene-one enzyme relationship was established for the first time in
-
Neurospora crassa
-
Salmonella typhimurium
-
Escherichia coli
-
Diplocococcus pneumoniae
A.
Neurospora crassa
One gene-one enzyme relationship was intially proposed by Beadle and Tatum and based on the experiments conducted on Neurospora crassa. They were awarded by Nobel Prize in 1958.
All enzymes of TCA cycle are located in the mitochondrial matrix except one which is located in inner mitochondrial membranes in eukaryotes and in cytosol in prokaryotes. This enzyme is
-
lactate dehydrogenase
-
isocitrate dehydrogenase
-
malate dehydrogenase
-
succinate dehydrogenase
D.
succinate dehydrogenase
Succinate dehydrogenase enzyme is present on inner membrane of mitochondria and catalysed the oxidation of succinate to fumarate.
An organic substance bound to an enzyme and essential for its activity is called
-
coenzyme
-
holoenzyme
-
apoenzyme
-
isoenzyme
A.
coenzyme
Coenzyme is an organic nonprotein molecule that associates with an enzymes molecule in catalysing biochemical reactions. It usually participates in the substrate-enzyme interaction by donating or accepting certain chemical groups.
Apoenzyme is an inactive enzyme that must associate with a specific cofactor molecule in order to function.
Isoenzyme or isozyme is one of the several forms of an enzyme that catalyse the same reaction but differ from each other in such properties as substrate affinity and maximum rates of enzymes-substrate reaction.
What is the role of NAD+ in cellular respiration?
It functions as an enzyme.
It functions as an electron carrier.
It is the final electron acceptor for anaerobic respiration.
It is a nucleotide source for ATP synthesis.
B.
It functions as an electron carrier.
In cellular respiration, NAD+ act as an electron carrier.
Fixation of one CO2 molecule through the Calvin cycle requires.
1 ATP and 2NADPH2
2 ATP and 2NADPH2
3 ATP and 2NADH2
2 ATP and 1 NADPH2
C.
3 ATP and 2NADH2
2 ATP is required during conversion of PGA to 1,3 diphosphoglyceric acid and 1 ATP during conversion of glyceraldehyde phosphate to ribulose biphosphate. 2NADPH2 molecules are utilised for converting 1,3 diphosphoglyceric acid to glyceraldehyde phosphate.
Match the storage products listed under column I with the organism given under column II, choose the appropriate option from the given options
| Column I | Column II | ||
| A | Glycogen | 1 | Sargassum |
| B | Pyrenoids | 2 | Nostoc |
| C | Laminarin and mannitol | 3 | Polysiphonia |
| D | Floridean starch | 4 | Spirogyra |
| 5 | Agaricus | ||
A B C D 3 4 1 5 A B C D 4 3 5 2 A B C D 5 4 1 3 A B C D 2 1 4 3
C.
| A | B | C | D |
| 5 | 4 | 1 | 3 |
In which of the following haemocyanin pigment is found?
Lower invertebrates
Echinodermata
Insecta
Annelida
A.
Lower invertebrates
In molluscs, the blood is colourless, often having copper containing blue respiratory pigment called haemocyanin.
Which one out of (a) to (d) given below correctly represents the structural formula of the basic amino acid?
D.
Basic amino acids have an additional amino group with forming amides thus, they are diaminomonocarboxylic acids, e.g., lysine arginine, etc.
Which one of the following structural formula of two organic compounds is correctly identified along with its related function?
B-uracil – a component of DNA
A-triglyceride – major source of energy
A-lecithin – a component of cell membrane
B-adenine – a nucleotide that makes up nucleic
C.
A-lecithin – a component of cell membrane
Lactose is composed of
Glucose + Glucose
Glucose + Galactose
Glucose + Fructose
Fructose + Galactose
B.
Glucose + Galactose
Lactose is popularly known as milk sugar. It is a disaccharide composed of one molecule of glucose and one molecule of galactose.
Deficiency of vitamin -B12 causes
Cheilosis
Thalassemia
Beri-Beri
Pernicious Anaemia
D.
Pernicious Anaemia
The deficiency (hypovitaminosis) of vitamin-B12 or cyanocobalamine causes pernicious anaemia, demyelination of nerve fibres and glossitis (inflammation of tongue).
Nucleotides are formed by
purine, sugar and phosphate
purine, pyrimidine and phosphate
purine, pyrimidine sugar and phosphate
pyrimidine, sugar and phosphate
C.
purine, pyrimidine sugar and phosphate
The nucleotide is formed by the union of a phosphate group with a nucleoside. A nucleoside in fact contains a sugar molecule along with an organic nitrogenous base. Thus, a nucleotide contains an organic nitrogenous base (purine or pyrimidine) along with a sugar molecule and a phosphate group, Nucleoside = Sugars molecule + organic nitrogenous based on Nucleotide = Nucleoside + Phosphate group.
Match the enzyme in column I with its function in column II and choose the correct option.
| Column I | Column II |
| A. Beta-Galactosidase | 1. Joining of DNA fragments |
| B. Permease | 2. Peptide Bond Formation |
| C. Ligase | 3. Hydrolysis of Lactose |
| D. Ribozyme | 4. Increases permeability to Beta-galactosidase |
A-2, B-1, C-4, D-3
A-3, B-2, C-1, D-4
A-2, B-4, C-1, D-3
A-1, B-2, C-4, D-3
B.
A-3, B-2, C-1, D-4
Beta-gal, coded by Z structural gene is primarily responsible for the hydrolysis of the diasaccharide lactose into its monomeric units, galactose and glucose.
Which of the following represent uridylic acid?
Uracil + Ribose
Uridine + Phosphoric acid
Uracil + Phosphoric acid
Uridine + Ribose + Phosphoric acid
D.
Uridine + Ribose + Phosphoric acid
It is a nucleoside of uridine formed in hydrolysis of RNA
Consider the following statements with two blanks X and Y and select the option which correctly fills up these blanks.
In the centre of the intervertebral disc, a soft area is present called ….X….....
Which is supposed to be remnant of …. Y....
X-Nucleus pulposus , Y-Nerve cord
X-Centrum, Y-Noto cord
X-Nucleus pulposus, Y-Noto cord
X-Centrum, Y-Nerve cord
C.
X-Nucleus pulposus, Y-Noto cord
It is a gel-like substance in the middle of spinal cord. It represents the ramnant of noto cord.
Given below is the chemical formula of
![]()
Palmitic Acid
Glycerol
Galactose
Stearic acid
A.
Palmitic Acid
Jacob and Monod name some enzymes as allosteric whose activity is regulated by
End product
Substrate
by product
Coenzyme
A.
End product
Aliosteric modulation or feedback inhibition of enzymes is influenced by end product. It was shown by Jacob and Monod 1961 through lac operon in E.coil.
Adenosine triphosphate was discovered by
Jack Lipman
A Bloor
Karl Lohmann
Emil Fisher
C.
Karl Lohmann
Adenosine Triphosphate (ATP) was discovered by Karl Lohmann in 1929 from muscles.
Keeping is viewed the structure of cell membrane, which one of the following statements is correct with respect to the movements of liquid and proteins from one liquid monolayer to the other (flip-flop movement).
While proteins can flip flop, liquids can not
Neither lipids, nor proteins can flip flop
Both lipids and proteins can flip flop
While lipids can rarely flip-flop, proteins can not
D.
While lipids can rarely flip-flop, proteins can not
The flip-flop movement refers to the transfer of lipid molecules from one side of the bilayer to the other, Enzymes are concerned with the enzymatic activity of the membrane.
Match the biological molecule listed under column I with their biological function listed under column II. Choose the answer which gives correct combination of alphabet of the two columns.
| Column I (Biological Molecule) | Column II (Function) |
| A. Glycogen | 1. Hormone |
| B. Globulin | 2. Biocatalyst |
| C. Steroid | 3. Antibody |
| D. Thrombin | 4. Storage Product |
A-3, B-2, C-4, D-1
A-4, B-2, C-1, D-3
A-2, B-4, C-3, D-1
A-4, B-3, C-1, D-2
D.
A-4, B-3, C-1, D-2
Glycogen is stored in the liver and muscle of animals. All steroid hormones are derived from cholesterol. Immunoglobulin is an antibody that freely circulates in blood plasma. Thrombin brings about the conversion of fibrinogen to fibrin during blood clotting mechanism.
The enzyme hexokinase which catalysis glucose to a glucose-6-phosphate in glycolysis is inhibited by glucose-6-phosphate. This is an example of
I. Competitive inhibition
II. non-competitive inhibition
III. feedback allosteric inhibition
Which of the above statements is/are correct?
I and II
I and III
Only III
All of these
C.
Only III
Hexokinase catalyses the phosphorylation of hexose in general and is found in all cells that metabolise glucose. It has low Km (high affinity, strong bonding) with glucose in such a way that it is active at low glucose concentration. It shows allosteric feed beat inhibition by to product glucose-6-phosphate.
Glycogenolysis involves
Conversion of sugar into glycogen
Oxidation of Sugar
Conversion of glycogen into sugar
Conversion of glycogen into fat
C.
Conversion of glycogen into sugar
Glycogenesis conversion of sugar to glycogen. Glycogenolysis conversion of glycogen to sugar.
An example of the competitive inhibition of an enzyme is the inhibition of
Succinic dehydrogenase by malonic acid
Cytochrome oxidase by cyanide
Hexokinase by glucose-6-phosphate
Carbonic anhydrase by carbon dioxide
A.
Succinic dehydrogenase by malonic acid
Classical example of competitive inhibition is reduction of activity of succinate dehydrogenase by malonate, oxaloacetate and other anions. In competitive inhibition, a competitive inhibitor which has the resemblance with substrate molecule, competes with the substrate for the active site of an enzyme. While the inhibitor (1) occupies the active site, it prevents binding of the substrate to the enzyme.
In the sieve elements, which one of the following is the most likely function of P-proteins?
Deposition of callose on sieve plates
Providing energy for active translocation
Autolytic enzymes
Sealing off mechanism on wounding
D.
Sealing off mechanism on wounding
In most angiospermic plants, sieve tube elements of phloem, are P-proteins. Later is found in all dictos and in many monocots but it is absent in gymnosperms and cryptogams. The main function of P-proteins is in sealing off damaged sieve elements by plugging up sieve plate pores.
Two cells A and B are contiguous. Cell A has osmotic pressure 10 atm, turgor pressure-7 atm and diffusion pressure deficit 3 atm. Cell B has osmotic pressure 8 atm, turgor pressure 3 atm and diffusion pressure deficit 5 atm. The result will be
Movement of water from cell B to A
No movement of water
The equilibrium between the two
Movement of water from cell A to B
D.
Movement of water from cell A to B
The water moves from lower DPD to higher DPD.
Which of the following amino acids is not optically active?
Glycine
Valine
Leucine
Isoleucine
A.
Glycine
Glycine is the simplest and smallest amino acid. A compound is said to be optically active, when a chiral or asymmetrical carbon (ie, carbon attached to four different groups or atoms) is present. Since, chiral carbin is absent in glycine, it does not show optical activity. Other amino acids such as Valine, Leucine and Isoleucine etc contains atleast one chiral carbon, therefore, are optically active.
Which of the following vitamins is water soluble as well as an antioxidant?
Vitamin- B1
Vitamin-A
Vitamin-D
Vitamin-C
D.
Vitamin-C
| Vitamin | Function | Source |
| I. Water soluble | ||
| (a) Vitamin- B complex | ||
| Vitamin- B1 | Anti beri-beri | Yeast, whole grains |
| Vitamin- B2 | Maintenance of oral mucosa | Milk, egg |
| (b) Vitamin- C | Antioxidant help in collagen and bone formation | Citrus fruits |
| II. Fat soluble | ||
| (a) Vitamin- A | Promotes normal vision | Carrot, cabbage |
| (b) Vitamin- D | Maintenance of Ca and P level in body | Cod and shark liver oil |
| (c) Vitamin- E | Antioxidant | Green vegetables, oil |
| (d) Vitamin- K | Anti= haemorrhagic | Green vegetables |
Which one is component of Ornithine cycle?
Ornithine, citrulline and alanine
Ornithine, citrulline and arginine
Amino acids are not used
Ornithine, citrulline and fumaric acid
B.
Ornithine, citrulline and arginine
Ornithine cycle or Krebs- Hanseleit cycle or urea cycle was dicovered by Hans Krebs and Kurt Hanseleit. It takes place in liver cells for synthesis of urea. The main componet of ornithine cycle are arginine, ornithine and citrulline.
Which compound has a very important role in prebiotic evolution?
Sulphur dioxide
Nitric oxide
Methane
Sulphur trioxide
C.
Methane
JBS Haldane (1920) used the term 'Prebiotic soup' or 'hot dilute soup of organic substances' for oceanic water containing mixture of simple organic compounds. Methane (CH4) was probably the first organic compound and hydrogen cyanide was formed later.
Which one is component of Ornithine cycle?
Ornithine, citrulline and alanine
Ornithine, citrulline and arginine
Amino acid are not used
Ornithine, citrulline and fumaric acid
B.
Ornithine, citrulline and arginine
Ornithine cycle or urea cycle or Krebs- Henseleit cycle was discovered by Han Krebs and Kurt Henseleit. It takes place in liver cells and the main components are arginine, ornithine and citrulline.
Paraffin wax is
ester
acid
monohydric alcohol
cholesterol
A.
ester
Waxes are esters formed between a long chain alcohol and saturated fatty acids. It is pliable and soft when warm but hard and water resistant when cold.
Steroids are complex compounds commonly found in cell membrane and animal hormone. Eg, Cholesterol, it reinforces the structure of cell membrane in animal cells.
Protein present in silk fibre is
caesin
keratin
elastin
fibroin
D.
fibroin
Silk fibre is formed of two proteins namely fibroin and sericin.
Which vitamin should not be stored?
Calciferol
Retinol
Niacin
Ascorbic acid
D.
Ascorbic acid
Vitamin- C (ascorbic acid) has virucidal property. It is water soluble vitamin, ie, it dissolves in water and its excess amount is excreted in urine.
Which one is the sweetest suagr?
Glucose
Fructose
Sucrose
Maltose
B.
Fructose
Fructose is the sweetest sugar. It is found in sweet fruits and in honey.
Nucleotide are building blocks of nucleic acids, nucleotide is a composite molecule formed by
(base-sugar-phosphate)n
base-sugar-OH
base-sugar-phosphate
sugar-phosphate
C.
base-sugar-phosphate
Nucleotides are the building blocks of nucleic acids (DNA/ RNA). A single nucleotide comprises of-
(i) phosphate molecule
(ii) a five carbon sugar (either ribose or deoxyribose)
(iii) a purine (adenine or guanine) or a pyrimidine (thymine or cytosine or uracil) nitrogenous base.
Nucleoside = Base + Sugar
Nucleotide = Base + Sugar + Phosphate
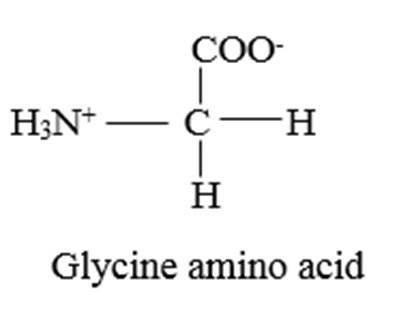
Which of the following is the simplest amino acid?
Tyrosine
Asparagine
Glycine
Alanine
C.
Glycine
Proteins are the polymers of amino acids in which amino acids are joined by peptide bonds. Glycine has the simplest structure.
Enzymes, vitamins and hormones can be classified into a single category of biological chemicals, because all of these
enhance oxidative metabolism
are conjugated proteins
are exclusively synthesized in the body of a living organism as at present
help in regulating metabolism
D.
help in regulating metabolism
Enzymes, vitamins and hormones are classified into a single category of biological chemical because all of them help in regulation of metabolism.
Enzymes are a proteinaceous catalyst produced by cell and are responsible for high rate and specificity of one or more inter/ intra cellular biochemical recations.
Vitamin is an organic substance, synthesized by plants (except Vitamin- D).
Hormones are chemical messengers which on secretion bring about a specific and adaptive physiological response.
In DNA, which is absent
Adenine
Thymine
Guanine
Uracil
D.
Uracil
Adenine, Guanine, Cytosine and thymidine are the bases of DNA, while Adenine, Guanine, Cytosine and uracil are the component of RNA.
Which is called soluble RNA
r-RNA
t-RNA
m-RNA
hn-RNA
B.
t-RNA
tRNA is known as the soluble RNA as they are soluble in 1M-NaCl. Hence they are also known as soluble RNA.
Micro-nutrients are
less important in nutrition than macronutrients
as important in nutrition as macro-nutrients
may be omitted from culture media without any detrimental effect on the plant
called micro because they play only minor role in nutrition
B.
as important in nutrition as macro-nutrients
Micro-nutrients are the minerals/elements which are required in less amount by the plants and thus are present in less quantity in the plants but these are as important as the macro-nutrients. Examples of micro-nutrients iron (Fe), manganese (Mn), copper (Cu), zinc (Zn), molybdenum (Mo), boron (B) and chlorine (Cl).
Carrier of fluorosis is
CO2
chlorine
nitrogen
water
D.
water
Fluorosis is a disease caused by the consumption of water with excess fluoride or fluorine.
Non-genetic RNA is of
two types
three types
only one type
none of these
B.
three types
Genetic RNA are those which have the property to replicate on its own such RNA are absent in human but they are present in some plant and microorganism.
However there are 3 major types of non- genetic RNA in human
(i) Ribosomal RNA (r-RNA)
(ii) Transfer RNA (t-RNA)
(iii) Messenger RNA (m-RNA)
A nucleotide is a molecule consisting of a
hexose sugar, phosphorus and albumen
phosphorus, iron and calcium
phosphate, 5-carbon sugar and nitrogen base
RNA and glucose
C.
phosphate, 5-carbon sugar and nitrogen base
A nucleotide is a nucleoside (sugar + nitrogen base) with phosphate group.
Thus,
nucleoside = sugar + nitrogenous base and nucleotide = nucleoside + phosphate group
Non-protein part of enzyme is known as
holoenzyme
apoenzyme
prothetic group
none of these
C.
prothetic group
The enzyme complex (holoenzyme) is made up of two parts i.e., protein part (apoenzyme) and non-protein part (cofactor). Cofactor is of three types-
(i) prosthetic group
(ii) coenzymes
(iii) metal ions
Holoenzymes = Apoenzyme + Prosthetic group (Enzyme complex) (Protein part) (Non-protein part).
Carbohydrates, the most abundant biomolecules on earth, are produced by:
all bacteria, fungi and algae
fungi, algae and green plant cells
some bacteria, algae and green plant cells
viruses, fungi and bacteria
C.
some bacteria, algae and green plant cells
Some photosynthetic bacteria such as Rhodopseudomonas can prepare carbohydrates.
But during this type of food synthesis O2 is not evolved because in this case hydrogen donor is other than H2O.
Algae (green and blue green) and all green plant cells prepare their food (carbohydrate) through photosynthesis. Here, hydrogen ions are donated by water molecules by the process of photolysis of water i.e., O2 is released during this type of food synthesis.
Which one is antioxidant vitamin?
Vitamin- D
Vitamin- E
Vitamin- B
Vitamin- K
B.
Vitamin- E
Antioxidant vitamins are Vitamin C, Vitamin E and - carotene. They are so called as they inactive oxygen free radicals. Free radicals are highly reactive particles that carry an unpaired electron [e-], they damage cell membranes, DNA, other cellular structures and contribute to formation of artery narrowings and atherosclerosis.
A nucleoside is
base + sugar
base + phosphate
sugar+ phosphate
base + sugar+ phosphate
A.
base + sugar
Base+ sugar form a nucleoside and base+ sugar + phosphate group combine together to give a nucleotide.
Which is a typical example of 'feedback inhibition'?
Allosteric inhibition of hexokinase by glucose-6-phosphate
Reaction between succinic dehydrogenase and succinic acid
Cyanide and cytochrome reaction
Sulpha drugs and folic acid synthesizer bacteria
A.
Allosteric inhibition of hexokinase by glucose-6-phosphate
Feedback inhibition is a form of enzyme regulation whereby products prevent product formation by binding to an allosteric site and inhibiting enzyme activity.
Identify the given structure
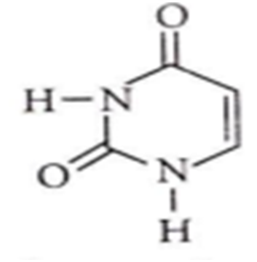
Adenylic acid
Uracil
Cholesterol
Adenosine
B.
Uracil
Uracil is one of the four nucleobases in the nucleic acid of RNA. It binds to adenine via two hydrogen bonds in RNA. It is replaced by thymine in case of DNA.
Which one of the following is an incorrect combination
Adenine, thymine, cytidine
Adenine, cytosine, thymine
Guanine, thymine, uracil
Cytosine, uracil, guanine
A.
Adenine, thymine, cytidine
Adenine (A), guanine (G), cytosine (C), thymine (T) and uracil (U) are all nitrogenous bases, whereas cytidine is a nucleoside i.e., a combination of a nitrogenous base (cytosine) with a pentose sugar (ribose).
Assertion: Secondary metabolites are produced in small quantities and their extraction from the plant is difficult and expensive.
Reason: Secondary metabolites can be commercially produced by using tissue culture technique.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
B.
If both assertion and reason are true but reason is not the correct explanation of assertion.
Secondary metabolites are biosynthetically derived from primary metabolites but more limited in distribution in plant kingdom, being restricted to a particular taxonomic group. Secondary metabolites are accumulated by plants in smaller quantities than are primary metabolites. Also, they are synthesised in specialised cell types and at distinct developmental stages, making their extraction and purification difficult and expensive. By the culture media using tissue culture techniques, secondary metabolites can be produced on a large scale.
Assertion : Carbohydrates are more suitable for the production of energy in the body than proteins and fats.
Reason : Carbohydrates can be stored in the tissues as glycogen and can be used for the production of energy, whenever necessary.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false
If both assertion and reason are false
B.
If both assertion and reason are true but reason is not the correct explanation of assertion
Carbohydrates are more suitable for the production ofenergy in the body than proteins and fats, because carbohydrate molecules contain relatively more oxygen than the others, and consequently, require less molecular oxygen for their oxidation. In other words, for each litre of oxygen consumed, carbohydrates yield far more energy than proteins or fats. Carbohydrates are also stored in the. tissues as glycogen for use in the production of energy, when necessary.
Which of the following are a group of micronutrients for plants
Fe, Mn, Cu, Mo, Zn
Fe, Mn, Cu, O, C
Cu, B, Cl, Fe, Ca
Ca, Mg, Fe
A.
Fe, Mn, Cu, Mo, Zn
Micronutrients are elements needed in small amounts (less than 10 mmolekg of dry matter). These are 8 in number and include iron, manganese, copper, molybdenum, zinc, boron, chlorine and nickel.
Which one of the following vitamins is antihaemorrhagic
Vitamin B12
Vitamin C
Vitamin B5
Vitamin K
D.
Vitamin K
Anti-haemorrhagic vitamins are those substances which promote haemostasis or stop bleeding. Vitamin K is one of them. Vitamin K affects the clotting mechanism by being essential for the production of four distinct clotting factors: prothrombin, factors VII, IX and X.
Assertion: Deficiency symptoms of N, K and Mg are first visible in the senescent leaves.
Reason: Biomolecules containing these elements are broken down in the older leaves, making these elements available for mobilising to younger leaves.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
A.
If both assertion and reason are true and reason is the correct explanation of assertion.
The parts of the plants that show the deficiency symptoms also depend on the mobility of the element in the plant. For elements that are actively mobilised within the plants and exported to young developing tissues, the deficiency symptoms tend to appear first in the older tissues. For example, the deficiency symptoms of nitrogen, potassium and magnesium are visible first in the senescent leaves. In the older leaves, biomolecules containing these elements are broken down, making these elements available for mobilising to younger leaves.
Which one of the following depresses brain activity and produces feelings of calmness, relaxation and drowsiness?
Morphine
Valium
Amphetamines
Hashish
B.
Valium
Valium is a benzodiazephine (sedative) that gives a feeling of relaxation, calmness or drowsiness in the body.
Morphine is the main opium alkaloid that depresses respiratory centre and contributes to the fall in blood pressure.
Amphetamines are synthetic drugs and are stimulant in nature.
Hashish is a hallucinogen.
You are required to draw blood from a patient and to keep it in a test tube for analysis of blood corpuscles and plasma. You are also provided with the following four types of test tubes. Which of these will you not use for the purpose
Test tube containing calcium bicarbonate
Chilled test tube
Test tube containing heparin
Test tube containing sodium hydroxide
C.
Test tube containing heparin
Clotting of collected blood can be prevented by
1) coating test tubes with silicon (which produce non wettable surface similar in its smoothness to endothelial lining of blood vessels).
2) adding chelating agents (includes trisodium citrate, sodium oxalate and sodium EDTA) which remove calcium which is important for blood coagulation, and prevent blood clotting.
3) adding heparin, most powerful anticoagulant which acts indirectly by activating plasma antithrombin III. Heparin is effective both in vivo and in vitro. Whereas the option a, b and d are effective in vitro. Heparinized blood is not suitable for blood counts (as it alters the shape of RBC's and WBC's which affects blood testing), Fragility testing and complement fixation tests.
Cattle fed with spoilt hay of sweet clover which contains dicumarol
are healthier due to a good diet
catch infections easily
may suffer vitamin K deficiency and prolonged bleeding
may suffer from beri-beri due to deficiency of vitamin B1
C.
may suffer vitamin K deficiency and prolonged bleeding
Dicumarol is an anticoagulant found in spoilts sweet clover causes hemorrhage and other symptoms of bleeding disorder by disrupting vitamin K metabolism and preventing the activation of prothrombin and certain other clotting factors by the liver.
Enzymes, vitamins and hormones can be classified into a single category of biological chemicals because of all of these
enhance oxidative metabolism
are conjugated proteins
are exclusively synthesised in the body of a living organism as at present
help in regulating metabolism.
D.
help in regulating metabolism.
Enzymes are protein that, in small amounts, speed up the rate of a biological reactions and help in regulating metabolism. Hormones are also metabolic regulator and help in stimulation or inhibition of one or more physiological processes. Vitamines are accessory food factors which are required in small quantity for controlling metabolism and body functioning.
Assertion: Competitive inhibitor is also called as substrate analogue.
Reason: It resembles the enzymes in structure.
If both assertion and reason are true and reason is the correct explanation of assertion
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false
If both assertion and reason are false.
C.
If assertion is true but reason is false
Competitive inhibition is the inhibition of enzyme activity by the presence of a chemical that competes with the substrate for binding to the active site of the enzyme. The inhibitor chemical is called substrate analogue or competitive inhibitor. It resembles the substrate in structure and gets bound up to the active site of the enzyme without getting transformed by the latter.
An example of competitive inhibition ofan enzyme is the inhibition of
succinic dehydrogenase by malonic acid
cytochrome oxidase by cyanide
hexokinase by glucose-6-phosphate
carbonic anhydrase by carbon dioxide
A.
succinic dehydrogenase by malonic acid
An example of competitive inhibition of an enzyme is the inhibition of succinic dehydrogenase by malonic acid. lt is the simple type of competitive inhibition. A competitive inhibitor resembles the substrate and binds to the active site of the enzyme. The substrate is then prevented from binding to the same active site.
Which of the following contain -1, 4 linkage?
Maltose
Sucrose
Lactose
Fructose
C.
Lactose
Lactose or milk sugar (carbohydrate) is reducing sugar formed through - 1, 4 condensation between galactose and glucose. Lactose does not occur in nature except as a product of the mammary gland. It is highest in human milk as compared to that of cow, buffalo and goat. Lactose is a product of glucose, fructose and galactose.
Maltose or malt sugar is a disaccharide formed from two units of glucose joined with an (14) bond. It is found in germinating grain, in a small proportion in corn syrup and forms on the partial hydrolysis of starch.
Sucrose or table sugar is a disaccharide where a molecule is composed of two monosaccharides, glucose and fructose. It is produced naturally in plants, from which table sugar is refined. It is extracted and refined from either sugar cane or sugar beet.
Fructose or fruit sugar is a simple ketonic monosaccharide found in many plants, where it is often bonded to glucose to form disaccharide sucrose.
Which statement is true?
Adenine has 4 nitrogen atoms.
Cytosine has 3 nitrogen atoms
Guanosine has 3 nitrogen atoms
Uracil has 5 nitrogen atoms
B.
Cytosine has 3 nitrogen atoms
Cytosine is one of the four main bases found in DNA and RNA. It is a pyrimidine derivative, with a heterocyclic aromatic ring and two substituents attached. It has 3 Nitrogen atoms.

Adenine is a purine nucleobase used in forming nucleotides of the nucleic acids. It binds to thymine via 2 hydrogen bonds to assist in stabilizing the nucleic acid structure.

Guanosine is a purine nucleoside comprising guanine attached to a ribose ring via - Ng- glycosidic bond. It can be phosphorylated to become guanosine monophosphate (GMP), cyclic guanosine monophosphate (cGMP), guanosine diphosphate (GDP) and guanosine triphosphate (GTP).

Uracil is the nucleobase of RNA instead of thymine in DNA. It binds to adenine via two hydrogen bonds.

The figure given below shows three velocity substrate concentration curves for an enzyme reaction. What do the curves a, b, and c depict respectively

a-normal enzyme reaction,
b-competitive inhibition,
c-non-competitive inhibition.a-enzyme with an allosteric modulator added,
b-normal enzyme activity,
c-competitive inhibitiona-enzyme with an allosteric stimulator,
b-competitive inhibition added
c-normal enzyme reactiona-normal enzyme reaction,
b-non-competitive inhibitor added
c-allosteric inhibitor added
A.
a-normal enzyme reaction,
b-competitive inhibition,
c-non-competitive inhibition.
Enzymes or biocatalysts are proteinaceous substance that are capable of catalysing chemical reactions of biological systems without themselves undergoing any change. In the graph a shows normal enzyme reaction, graph h shows competitive inhibition and graph c shows non competitive inhibition. In competitive inhibition the inhibitor, which is a substrate analogue, combines reversibly to the free enzyme at the active site. In non competitive inhibition, the inhibitor binds to enzyme at a place other than substrate binding site. It results in destruction of enzyme activity
Which one of the following statements regarding enzyme inhibition is correct?
Competitive inhibition is seen when a substrate competes with an enzyme for binding to an inhibitor protein.
Competitive inhibition is seen when the substrate and the inhibitor compete for the active site on the enzyme
Non-competitive inhibition ofan enzyme can be overcome by adding large amount of substrate
None of these
B.
Competitive inhibition is seen when the substrate and the inhibitor compete for the active site on the enzyme
In competitive inhibition, an inhibitor that resembles the normal substrate binds to the enzyme, at the active site, and prevents the substrate from binding.
Non-competitive inhibition is a type of enzyme inhibition where the inhibitor reduces the activity of the enzyme and binds equally well to the enzyme whether or not it has already bound the substrate.
In an experiment freshly hatched larvae of an insect (Khapra beetle) were reared on a basal diet (complete diet without cholesterol) with increasing amounts of cholesterol. Results obtained are shown in the graph given in the table :
The graph indicates that

cholesterol is an essential dietary requirement of khapra beetle
growth of khapra beetle is directly proportional to cholesterol concentration
cholesterol concentration of 2 µg/g diet is the optimum level
growth of khapra beetle is inhibited when cholesterol concentration exceeds 5 g/g diet
A.
cholesterol is an essential dietary requirement of khapra beetle
According to the graph, the growth of the insect takes place as the amount of the cholesterol increase and at 6 g cholesterol/g the growth get static. If the growth rate would have been directly proportional to the cholesterol concentration then the graph would have been straight line.
Option 3 is wrong as the optimum level is 6 µg/g cholesterol concentration.
An example of competitive inhibition of an enzyme is the inhibition of:
succinic dehydrogenase by malonic acid
cytochrome oxidase by cyanide
hexokinase by glucose-6-phosphate
carbonic anhydrase by carbon dioxide
A.
succinic dehydrogenase by malonic acid
In competitive inhibition, an inhibitor that resembles the normal substrate binds to the enzyme, usually at the active site and prevents the substrate from binding.
An example of competitive inhibition of an enzyme is the inhibition of succinic dehydrogenase by malonic acid. It is the simple type of competitive inhibition. A competitive inhibitor resembles the substrate and binds to the active site of the enzyme. The substrate is then prevented from binding to the same active site.
Antibiotic flavicin is obtained from
Aspergillus flavus
Aspergillus clavatum
Streptomyces grieseus
Streptomyces fradiae
A.
Aspergillus flavus
Aspergillus flavus is the fungi which grows on plants such as peanut plant. It is used in the production of the antibiotic Flavicin. It is also used to produce the carcinogenic substance Aflatoxin.
Antifeedant property occurs in
nicotine
azadiractin
rotenone
cinerin
B.
azadiractin
Antifeedants are organic compound produced by plants to inhibit attack by insects and grazing animals. These chemical compounds are typically classified as secondary metabolites in that they are not essential for the metabolism of the plant, but instead confer longevity. Antifeedants exhibit a wide range of activities and chemical structures as biopesticides.
Azadirachtin are extract of neem tree, it has several effects on phytophagous insects and is thought to disrupt insect molting by antagonizing the effects of ecdysteroids. This effect is independent of feeding inhibition, which is another observed effect of the compound.1,10 The antifeedant/repellent effects are dramatic, with many insects avoiding treated crops, although other chemicals in the seed extract, such as salanin, have been shown to be responsible for these effects.
Lipids are insoluble molecules are :
neutral
Zwitter ions
hydrophobic
hydrophilic
A.
neutral
Lipid is a term used to describe a group of substances in cell, characterized by their solubility in organic solvents such as ether and benzene. These are insoluble in water, because they contain hydrophobic fatty acid chain.
Which of the following is the simplest amino acid?
Glycine
Alanine
Tyrosine
Asparagine
A.
Glycine
Amino acid is the monomer unit of protein. Glycine is the simplest amino acid as it contains one amino group and one carboxylic group. The structural formula of Glycine is

Carbohydrates, ingested in the diet, are hydrolyzed by the enzyme :
pepsin
cellulose
- amylase
glycosidase
C.
- amylase
The principal of carbohydrate in diet is starch i.e., the carbohydrates, ingested in the diet, are hydrolyzed by the enzyme called -amylase. This enzyme is found in saliva and intestinal juice. It's main function is to break down glycosidic bonds between glucose units.
Pepsin is an endopeptidase that breaks down proteins into smaller peptides. It is produced in the stomach and is one of the main digestive enzymes in the digestive systems of humans and many other animals.
Cellulose is the main constituent of cell wall in plants. It provides rigidity or stiffness to plants. It is the abundant organic compound on Earth.
Glucosidases are enzymes involved in breaking down complex carbohydrates such as starch and glycogen into their monomers. Their main function is to catalyze the cleavage of individual glucosyl residues from various glyco- conjugates including alpha or beta linked polymers of glucose.
The vitamin present in Coenzyme is
B2
niacin
pyridoxine
pantothenic acid
A.
B2
Vitamin B2 in the form of riboflavin 5'-phosphate sometimes called flavinmononucleotide (FMN).
Quaternary structure of protein
consists of four subunits
may be either or
is unrelated to two functions of the protein
is dictated by the primary structures of the individual subunits
D.
is dictated by the primary structures of the individual subunits
Proteins are linear polymers of amino acids. Many highly complex proteins consist of an aggregation of polypeptide chains, which are held together by hydrophobic interaction, and hydrogen, ionic bonds. Their precise arrangement constitutes the quaternary structure.
Identify the sulphur containing amino acid
proline
methionine
aspartic acid
tryptophan
B.
methionine
Amino acid is a molecule containing amino (-NH2) group a carboxyl (-COOH) group, a hydrogen atom and a side chain, all bonded together to a central carbon atom.

Methionine is the hydrophobic amino acid and consists of sulphur containing group. Alongwith methionine, cysteine consists of sulphur containing group.
Which of the following carbohydrates is not a disaccharide?
Maltose
Lactose
Sucrose
Galactose
D.
Galactose
Galactose is a monosaccharide.

Which of the following vitamins are fat soluble?
A, B, C, K
A, B, D, E
A, D, E, K
A, D, C, K
C.
A, D, E, K
Vitamins are naturally occurring organic substances, which are required in minute amount to maintain normal health and are to be supplied in food as they can not be synthesized by the organism (except vitamin-D). The fat soluble vitamins are A, D, E and K.
Which element is cause of itai 1/N desh itai disease
Hg
Cd
Pb
As
B.
Cd
Itai-itai disease was the name given to the mass cadmium poisoning of Toyama Prefecture, Japan, starting around 1912. The term 'itai-itai disease' was coined by locals for the severe pains people with the condition felt in the spine and joints.Cadmium poisoning can also cause softening of bones and kidney failure.
What is pro-enzyme?
A proenzyme (or zymogen) is an inactive enzyme precursor. It requires a biochemical change (such as a hydrolysis reaction revealing the active site, or changing the configuration to reveal the active site) for it to become an active enzyme. It is secreted by pancreas.
Name two sulphur containing and two basic amino acids.
There are two amino acids which contain a sulphur atom: cysteine and methionine.


Feedback inhibition of enzymes is affected by which ofthe following
Enzyme
Substrate
End products
Intermediate end products
C.
End products
Feedback inhibition is the phenomenon where the output of a process is used as an input to control the behavior of the process itself, oftentimes limiting the production of more product. Although negative feedback is used in the context of inhibition, negative feedback may also be used for promoting a certain process
Pellagra is caused due to deficiency of the vitamin
thiamine
niacinn
pyridoxin
biotin
B.
niacinn
Pellagra is a disease caused by low levels of niacin, also known as vitamin B-3. It's marked by dementia, diarrhea, and dermatitis, also known as “the three Ds”. If left untreated, pellagra can be fatal.
Which one is diaminodicarboxylic amino acid?
Cystine
Lysine
Cysteine
Aspartic acid
B.
Lysine
Lysine is a diaminodicarboxylic amino acid with the chemical formula HO2CCH(NH2)(CH2)4 NH2. This amino acid is an essential amino acid, which means that humans cannot synthesize it. Its codon are AAA and AAG.
Which one is the cofactor of carbonic anhydrase?
Iron (Fe)
Zinc (Zn)
Copper (Cu)
Magnesium (Mg)
B.
Zinc (Zn)
Many enzymes require the presence of an additional, nonprotein, cofactor. Some of these are metal ions such as Zn2+ (the cofactor for carbonic anhydrase), Cu2+, Mn2+, K+ and Na+. Some cofactors are small organic molecules called coenzymes. The vitamin-B are the precursors of coenzyme.
Vitamin-D is produced in human body in
muscles
nerves
skin
bone marrow
C.
skin
Vitamin-D is produced in the skin of vertebrates after exposure to ultraviolet light and occurs naturally in a small range of foods.
Which of the following is not a conjugated protein?
Peptone
Phosphoprotein
Lipoprotein
Chromoprotein
A.
Peptone
A conjugated protein is a protein that functions in interaction with other chemical groups attached by covalent bonds or by weak interactions, eg, lipoproteins, glycoproteins, chromoproteins, phosphoproteins, haemoproteins, flavoproteins, metalloproteins, phytochromes, cytochromes and opsin.
Which one is true for ATP?
ATP is a prosthetic part of an enzyme
ATP is an enzyme
ATP is the organic ions of enzyme
ATP is a coenzyme
D.
ATP is a coenzyme
Adenosine-triphosphate (ATP) is a multifunctional nucleotide used in cells as a coenzyme. It is often called the molecular unitof currency of intracellular energy transfer. ATP transports chemical energy within cells for metabolism. It is produced by photophosphorylation cellular respiration and used by enzymes and structural proteins in many cellular processes including biosynthetic reactions, motility and cell division.
Purines possess nitrogen at
1, 2, 4 and 6 position
1, 3, 5 and 7 position
1, 3, 7 and 9 position
1, 2, 6 and 8 position
C.
1, 3, 7 and 9 position
Purine is an organic nitrogenous base, sparingly soluble in water, that gives rise to a group of biologically important derivatives. Purine possess nitrogen at 1, 3, 7 and 9 position.

How many effective codons are there for the synthesis of twenty amino acids?
64
32
60
61
A.
64
Codons code for amino acids, there are 64 codons total and 3 codons do not code for any amino acids, as they function as stop codons. Hence, there are 61 effective codons for the synthesis of twenty amino acids.
Glucose and amino acids are reabsorbed in the
proximal tubule
distal tubule
collecting duct
loop of Henle
A.
proximal tubule
Proximal convoluted tubule of nephron is pivotal site for reabsorption of glucose, amino acids, Na+, K+ by active transport. Here, 80% of water is reabsorbed by passive transport.
Which one is imino acid?
Pepsin
Proline
Cysteine
Renin
B.
Proline
An imino acid is any molecule that contains both imino (>C=NH) and carboxyl (-COOH) functional groups. Eg, Proline and Hydroxy proline.
Methionine and cysteine are sulphur containing amino acids.
The lactase hydrolyses lactose into
glucose
glucose and galactose
fructose
glucose and fructose
B.
glucose and galactose
The enzyme lactase hydrolyses lactose (disaccharide) into glucose and galactose.
Lactose Glusoce + Galactose
Example of a typical homopolysaccharide is
lignin
suberin
inulin
starch
D.
starch
Polysaccharides are branched or unbranched polymer of monosaccharides. Homopolysaccharides contain a single type of monomers, eg, starch, glycogen, cellulose etc.
Choose the correct non-protein amino acid
hydroxyproline
hydroxylysine
cystine
y-amino butyric acid
D.
y-amino butyric acid
Gamma aminobutyric acid (GABA) is a naturally occurring amino acid that works as a neurotransmitter in your brain. Neurotransmitters function as chemical messengers.
Inulin is a polymer of
glucose
galactose
fructose
arabinose
C.
fructose
Inulin is a starchy substance found in a wide variety of fruits, vegetables and herbs, including wheat, onions, bananas etc. It is a polymer of fructose. It consists of 30 fructose units linked by 1-2 linkage.
Mannitol is
amino acid
amino alcohol
sugar alcohol
sugar acid
C.
sugar alcohol
Mannitol is a colourless sweet- tasting crystalline alcohol or sugar alcohol which is found in many plants and is used in various foods and medical products.
Structural lipids of cell membrane are
simple lipid
chromolipids
steroid
phospholipids
D.
phospholipids
Cell membrane (pasmalemma) is composed of proteins, lipids and some amount of carbohydrate. Membrane lipid is primarily phospholipid. It contains both polar and non- polar portion.
Which one of the following is polysaccharide?
Glycogen
Sucrose
Lactose
Maltose
A.
Glycogen
Polysaccharides are polymers of monosaccharides. Glycogen and starch are both polymer of -glucose. Glycogen found in liver and muscles store energy in mammals.

Which one of the following is not true for enzymes?
They act on a specific substrate
They are made up of fat and sugar
They act at a specific temperature
They act at a specific pH
B.
They are made up of fat and sugar
Enzymes or biocatalysts are exclusively proteinaceous substances capable of catalysing chemical reactions of biological systems without themselves undergoing any change. There are certain factors that affect enzymatic activity:
- Temperature
- pH
- Enzyme concentration
- Substrate concentration
- Presence of inhibitors or activators
Identify the polysaccharide with -glycosidic bond
starch
glycogen
sucrose
cellulose
D.
cellulose
Cellulose is the most important structural component of the cell wall of plants. It is a linear polymer of -D glucose units connected through -1, 4 glycosidic linkage.

Simple storage protein that coagulates upon heating but remains soluble in dilute salt solution is correctly exemplified by
globulin
albumin
histone
collagen
B.
albumin
Albumins are water soluble proteins which are coagulated by heat and precipitated by saturated salt solutions.
Globulins are insoluble in pure water and moderately concentrated salt solutions. These are coagulated by heat, while histones are not coagulated by heat.
Inulin is a type of
protein
carbohydrate
lipid
nucleic acid
B.
carbohydrate
Inulin is a soluble polysaccharide (carbohydrate) occurring as stored food material in many plants, such as members of compositae and in dahlia tubers. it is composed of 30 fructose units linked by -1, 2-linkages. Fructose is present as furanose ring in inulin.
Higher animals cannot synthesize few fatty acids which are very essential for their growth and deveiopment. These fatty acids are typically
saturated
cyclic
unsaturated
branched
C.
unsaturated
Essential fatty acids are poly-unsaturated fatty acids that our body can not synthesize and therefore, has to be provided through diet.
What is the substrate for lipase enzyme?
Protein
Carbohydrate
Lipid
Nucleic acid
C.
Lipid
Lipases represent a class of enzymes catalysing hydrolysis of esters of fatty acids and other lipids. They convert triglycerides (fats) into fatty acids, monoglycerides and glycerol.
What are ketone bodies?
Acetoacetic acid, acetone and beta-hydroxybutyric acid
Nicotinic acid, folic acid and ascorbic acid
Acetone, beta-hydroxybutyryl Co-A and acetoacetic acid
Acetic acid, acetone and beta-hydroxybutyric acid
A.
Acetoacetic acid, acetone and beta-hydroxybutyric acid
The acetyl Co- A produced by mitochondrial -oxidation of fatty acids enters the Krebs cycle to produce energy, but that is not the only fate of acetyl Co- A. In liver mitochondria, some acetyl Co- A is converted to acetoacetate, - hydroxy butyrate and acetone, collective called ketone bodies, which are transported to other tissues (e.g., brain, muscle or heart) where they are converted back to acetyl Co- A to serve as an energy source.
Transition state structure of the substrate formed during an enzymatic reaction is
transient but stable
permanent but unstable
transient and unstable
permanent and stable
C.
transient and unstable
The substrate binds to the enzyme at its active site forming an enzyme substrate complex. This complex formation is a transient and unstable phenomenon. Later, the product is released from the active site, which makes all other intermediate structural states unstable. Hence, stability is related to energy status of the molecule or the structure.
A phosphoglyceride is always made up of
only a saturated fatty acid esterified to a glycerol molecule to which a phosphate group is also attached
only an unsaturated fatty acid esterified to a glycerol molecule to which a phosphate group is also attached
a saturated or unsaturated fatty acid esterified to a glycerol molecule to which a phosphate group is also attached
a saturated or unsaturated fatty acid esterified to a phosphate group, which is also attached to a glycerol molecule
C.
a saturated or unsaturated fatty acid esterified to a glycerol molecule to which a phosphate group is also attached
Phosphoglycerides are esters of two fatty acids, phosphoric acid and a trifunctional alcohal glycerol.
A fat is formed of two kinds of smaller molecules, i.e., glycerol and fatty acids. Fatty acid molecules join together to glycerol by an ester linkage. A fatty acid has a long carbon skeleton, usually 16 or 18 carbon atoms in length. It there are no double bonds between carbon atoms composing the chain, then as many hydrogen atoms as possible are bonded to the carbon skeleton. Hence, this is known as saturated fatty acids. Unsaturated fatty acid has one or more double bonds.
Macromolecule chitin is
nitrogen containing polysaccharide
phosphorus containing polysaccharide
sulphur containing polysaccharide
simple polysaccharide
A.
nitrogen containing polysaccharide
Macromolecule chitin is a complex polysaccharide containing amino sugars and chemically modified sugars (e.g., glucosamine, N-acetyl galactosamine, etc). Polysaccharides are long carbohydrate molecules of monosaccharide units joined together by glycosidic bonds. They have a general formula Cx(H2O)Y.
The essential chemical components of many coenzymes are
proteins
nuclei acids
carbohydrates
vitamins
D.
vitamins
Coenzymes are organic molecules that are required by certain enzymes to carry out catalysis. They bind to the active site of the enzyme and participate in catalysis but are not considered substrates of the reaction. Essential chemical components of many coenzymes are vitamins eg, coenzyme Nicotinamide Adenine Dinucleotide (NAD) and NADP contain the vitamin niacin. Proteins, nucleic acids and carbohydrates are not enzymatic biomolecules.
One molecule of triglyceride is produced using
one fatty acid and one glycerol
one fatty acid and three glycerols
three fatty acids and three glycerols
three fatty acids and one glycerol
D.
three fatty acids and one glycerol
Alcohol have a hydroxyl group (-OH) and organic acids have a carboxyl group (-COOH). When alcohol and organic acids join, they form esters. The glycerol molecule has three hydroxyl group and each fatty acid has three carboxyl group. Hence, a triglyceride or triacylglyceride an ester is formed from the reaction between three fatty acids and one glycerol.
Glutenin is an important protein in
potato
wheat
soyabean
spinach
B.
wheat
Gluten is found in wheat endosperm. It is a type of tissue produced in seeds which is grounded to make flour. It is responsible for both providing nourishment to plant embryo during germination and affecting the elasticity of the dough therey, affecing the chewiness of baked wheat products. Gluten is actually composed of two different proteins, i.e., gliadin (a protamin protein) and glutenin (a glutelin protein). Soyabean contain soya protein, spinach and potatoes contains very less amount of protein.
Which one of the following is enriched with a non-reducing sugar?
Grapes
Germinating barley grains
Table sugar
Mother's milk
C.
Table sugar
Sucrose is a disaccharide. It is commonly known as sugar, table sugar, cane sugar or beet sugar. It is enriched with non- reducing sugars because it is a carbohydrate which is not oxidised by a weak oxidising agent in basic aqueaous solution, i.e., it does not generate any compounds containing an aldehyde group. Sugar found in other given options ore grapes glucose and fructose (in grapes), grains maltose in germinating barley and lactose in mother's milk are all enriched with a reducing sugar, i.e., they has an aldehyde group or are capable of forming one in solution through isomerism.
Which of the following statements is wrong for sucrose?
It is a disaccharide
It is a non-reducing sugar
It accumulates in the cytoplasm
It is comprised of maltose and fructose
D.
It is comprised of maltose and fructose
Sucrose is a disaccharide, mainly composed of fructose and glucose. It is a non-reducing sugar because it does not have a free aldehyde or ketone group. During the transportation of sucrose from source to sink through phloem it gets accumulated in the cytoplasm of the cell, after getting hydrolysed into glucose and fructose.
The protein component of a holoenzyme is known as
coenzyme
cofactor
prosthetic group
apoenzyme
D.
apoenzyme
Apoenzyme is a protein portion which combines with a cofactor, i.e., either coenzyme, metal ion activator or prosthetic group is collectively, called an active holoenzyme.
Km is
product
enzyme
constant
unit
C.
constant
Km acts as a Michaelis- Menten constant in the equation which is as follows-
where, Vmax = maximum rate achieved by the substrate concentration
Km = substrate concentration at which rate of reaction is half of the max. velocity.
S = substrate
Absorption of vitamin-B;» in human requires 'P' glycoprotein secreted from 'Q'. The correct choices of P and Q are
P - Extrinsic factor; Q - Stomach
P - Intrinsic factor; Q - Stomach
P - Intrinsic factor; Q - Small intestine
P - Exopolysaccharide; Q - Small Intestine
B.
P - Intrinsic factor; Q - Stomach
Intrinsic Factor (IF) also known as gastric Intrinsic Factor (GIF), is a glycoprotein produced by the parietal cells of the stomach. It is present in the gastric juice as well as gastric mucous membrane.
Upon entry into the stomach, vitamin-B12 becomes bound to haptocorrin (a glycoprotein.) The resulting complex enters the duodenum, where pancreatic enzymes digest haptocorrin. In the less acidic environment of the small intestine, B12 can then bind to intrinsic factor.
Which one of the following combination of all three fatty acids are essential for human beings?
Oleic acid, linoleic acid and linolenic acid
Palmitic acid, linoleic acid and arachidonic acid
Oleic acid, linoleic acid and arachidonic acid
Linoleic acid, linolenic acid and arachidonic acid
D.
Linoleic acid, linolenic acid and arachidonic acid
Fatty acids, such as linoleic, linolenic and arachidonic acids contain two or more cis carbon-carbon double bonds and are referred to as polyunsaturated fatty acids. These fatty acids are required nutrients for humans and must be part of a healthy diet.
They are termed essential fatty acids. These cannot be synthesised by human beings but are essential to human health. They must be consumed in adequate amounts specially in the form of ingested plant derived foods.
An allosteric inhibitor of the enzyme acts by binding to the
substrate
product
catalytic site of the enzyme
non-catalytic site of the enzyme
D.
non-catalytic site of the enzyme
The activity of certain enzymes, i.e. allosteric enzymes is regulated by compounds, which are not their substrates and which bind to the enzyme at specific sites away from the catalytic site.
They modify erizyme activity by producing a reversible change in the structure of the enzymes catalytic site. Allosteric inhibitors slow down the reaction rate by binding to the non-catalytic site of the enzyme.
Which one of the followings is correct for the trans membrane proteins in lipid bilayer?
They are absent in animal cells
They act as channel proteins
They are absent in plant cells
They are only externally located
B.
They act as channel proteins
Transmembrane proteins in lipid bilayer occur in many forms and are responsible for transferring specific solutes across cell membrane.
Channel proteins, from water - filled pores that extend across the lipid bilayer, when these pores are open, they allow specific solutes (usually inorganic ions of appropriate size and charge) to pass through them and there by cross the membrane.
Which of the following statements is/are correct regarding the effects of pH on enzyme catalysed reactions?
Direction of the reaction is influenced by [H+]
Ionisation state of dissociating groups on the enzyme is modified
Ionisation state of the substrate is modified.
Protein is not denatured with the change in pH
A.
Direction of the reaction is influenced by [H+]
B.
Ionisation state of dissociating groups on the enzyme is modified
C.
Ionisation state of the substrate is modified.
Most proteins and therefore enzymes are active only within a narrow pH range between 5 and 9. pH is defined as the -log[H+] i.e., the direction of the reaction is influnced by [H+]. It also effects the ionisation state ofsubstrate, amino acid residues, dissociating groups on the enzyme. Extremes of pH which is not optimal for enzyme catalysed reactions to take place leads to denaturation of proteins.
Which one from those given below represents the position of nitrogen in the 9-membered double rings of purines?
2, 4, 6 and 8
1, 3, 7 and 9
1, 4, 8 and 9
3, 5, 7 and 8
B.
1, 3, 7 and 9
Purines are 9-membered double rings with nitrogen at 1, 3, 7 and 9 positions. DNA has two types of purines namely Adenine (A) and Guanine (G).

Out of the given below statements describing the characteristics of various biomolecules, option for the incorrect one
Fatty acids have hydrocarbon chains that end in a carboxylic group
Starch consist of amylose and glycogen, which are formed by the condensation of -D-glucose.
Cellulose is fibrous polysaccharide of high tensile strength.
Cutin is a complex lipid produced by cross-esterification and polymerisation of hydroxy fatty acids.
B.
Starch consist of amylose and glycogen, which are formed by the condensation of -D-glucose.
Starch consist of two components amylose and amylopectin. Amylose is more soluble in water than amylopectin. They both are formed by the condensation of -D-glucose or pyranose forms.
Identify the correct match from the type of amino acids and their respective example.
Neutral - Proline (Pro)
Acidic - Asparagine (Asn)
Aromatic - Tryptophan (Try)
S-containing - Methionine (met)
A.
Neutral - Proline (Pro)
Proline is a heterocyclic amino acid neutral amino acids include alanine, glycine, etc.
When all the active sites of the enzyme are occupied, how does increasing the substrate concentration affect the rate ofreaction?
The rate of reaction will slow down
The rate of reaction will become constant, and shows no increase
The rate of reaction will increase with increasing substrate concentration
The rate of reaction will become reactive in such condition
B.
The rate of reaction will become constant, and shows no increase
An increase in substrate concentration when no active sites are available would not be able to speed up the initial rate of reacton and rate of reaction will become constant.
Which ofthe following unsaturated fatty acid possess four double bond?
Arachidonic acid
Linolenic acid
Oleic acid
Linoleic acid
A.
Arachidonic acid
Unsaturated acids possess one or more double bonds in their carbon chains. The general formula is CnH2n- 2xO2
Oleic acid = One double bond
Linoleric acid = Two double bonds
Linolenic acid = Three double bonds
Arachidonic acid = Four double bonds
Polysaccharides, polypeptides and polynucleotides have in common that they all
contain amino acid
are formed in condensation reactions
contain nitrogen
come out in acid soluble pool
B.
are formed in condensation reactions
All the polysaccharides, polypeptides and polynucleotides are formed by condensation reaction or dehydration synthesis process.
It is a chemical reaction in which two molecules or moieties (functional group) combine to form a larger molecule together with the loss of a small molecule.
Select the type of enzyme involved in the following reaction
S - G + S' S + S' - G
Lyase
Transferase
Hydrolase
Dehydrogenase
B.
Transferase
Transferase is a class of enzymes that catalyse the transfer of a group of atoms from one molecules to another.
Lyase is an enzyme that catalyzes the breaking of various chemical bonds by means other than hydrolysis and oxidation, often forming a new double bond or a new ring structure.
Hydrolase is an enzyme that catalyses the hydrolysis of a particular substrate.
Dehydrogenase is an enzyme that catalyses the removal of hydrogen atoms from a particular molecule, especially in ETC reactions.
Usually the activity of the enzymes is optimum at normal body temperature. At about 0C the activity of the enzyme is
minimum
maximum
Both (a) and (b)
None of these
A.
minimum
At very low temperature, i.e., about 0°C the activity of the enzyme is minimum.
Non-reducing sugars have
free CHO group and bound CO group
free CO group and bound CHO group
both CO and CHO free groups
neither free CO nor free CHO groups
D.
neither free CO nor free CHO groups
Non- reducing sugars lack free aldehyde or ketonic group and they do not reduce cupric ions of Benedict's or Fehling's solution to cuprous ions, e.g., sucrose.
Which vitamin should not be stored ?
Calciferol
Retinal
Niacin
Ascorbic acid
D.
Ascorbic acid
Ascorbic acid or Vitamin- C has virucidal property and in its excess amount is excreted in urine because it is water soluble.
Which enzyme converts glucose into alcohol ?
Zymase
Diastase
Invertase
Lipase
A.
Zymase
Glucose and fructose are both converted to ethanol and carbon dioxide in presence of zymase enzyme.
C6H12O6 2C2H5OH + 2CO2
Glucose/ Fructose Ethanol
Invertase enzyme converts sugar into glucose and fructose.
C12H22O11 + H2O C6H12O6 + C6H12O6
Sugar Glucose Fructose
Gastric juice consists of weak gastric lipase which converts some fats into monoglycerides and fatty acids. Bile salts of the bile break down fat droplets into many small ones by reducing the surface tension offat droplets. This procress is called emulsification. This increases lipase action on fat. Lipase is present in the pancreatic juice and intestinal juice. Pancreatic lipase is the principal enzyme for the digestion of fat.
Fat Emulsified fat
Emulsified Fatty acid + Diglyceride
Diglyceride Fatty acid + Monoglyceride
Monoglyceride Fatty acid + Glycerol
The enzyme, which combines with non-protein part to form a functional enzyme known as :
co-enzyme
holoenzyme
apoenzyme
prosthetic group
B.
holoenzyme
Apoenzyme is the proteinaceous part of an enzyme. The apoenzyme plus non- protein aceous part is called holoenzyme.
Co-enzyme is a non- protein compound that is necessary for the function of an enzyme.
Prosthetic group is a non- protein group, forming a part of or combined with a protein.
Major role of minor elements inside living organisms is to act as :
binder of cell structure
constituent of hormone
building blocks of important amino acids
cofactor of enzymes
D.
cofactor of enzymes
The micro-elements are required by plants in trace ammount (0.1 mg/gm dry matter). These are mostaly involved in the functioning of enzyme as co factors or metal activator.
भारत माता के प्रति नेहरू जी की क्या अवधारणा थी?
भारतमाता के प्रति नेहरूजी की अवधारणा यह थी कि हिन्दुस्तान के नदी और पहाड़, जंगल और खेत तो भारतामाता के अंग हैं ही, इसके साथ-साथ हिन्दुस्तान के लोग, जो सारे देश में फैले हुए हैं, ये ही लोग असल में भारतामाता हैं।
‘भारत माता की जय’ से मतलब इन्हीं लोगों की जय से है।
Mock Test Series
Sponsor Area
Sponsor Area