Sponsor Area
Biomolecules
The figure given below shows the conversion of a substrate to product by an enzyme. In which one of the four option (a-d), the components of reaction labelled as A, B, C and D are identified correctly?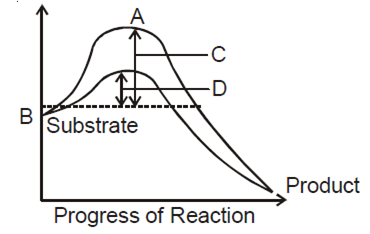
-
A
B
C
D
Potential energy
Transition state
Activation energy with enzyme
Activation energy without enzyme
-
A
B
C
D
Transition energy
Potential energy
Activation energy with enzyme
Activation energy with out enzyme
-
A
B
C
D
Potential energy
Transition energy
Activation energy with out enzyme
Activation energy with enzyme
-
A
B
C
D
Activation energy with enzyme
Transition energy
Activation energy with out enzyme
Potential energy
B.
|
A |
B |
C |
D |
|
Transition energy |
Potential energy |
Activation energy with enzyme |
Activation energy with out enzyme |
The amount of energy required to raise the energy of molecules at which chemical reaction can occur is called activation energy. Thus, acitvatgion energy is actually the energy required to form the transition state. Enzymes dramatically reduce the activation energy of a reaction, so that most molecules can easily get over the activation energy barrier and quickly turn into product. Simply we can say that activation energy of an enzyme catalysed reaction is lower than that of an uncatalysed reaction.
Some More Questions From Biomolecules Chapter
What are the contents of lactose ?
Give the names of two nucleotides.
What are carbohydrates ?
Give the names of two vitamin nucleotides.
What are the different types of carbohydrates ?
Where do you find the myoglobin in the body ?
What are monosaccharides ?
Give the names of two trace elements.
What is glycosidic bond ?
What are heterocyclic aminoacids ?
Sponsor Area
Mock Test Series
Mock Test Series





