Science Chapter 5 The Fundamental Unit Of Life
Sponsor Area
NCERT Solution For Class 9 About 2.html
Compare the properties of electron, proton and neutron.
| Particle | Nature of charge | Mass | Location |
| Electron | negtive -1.6 x 10-19C |
9.0 x10-31Kg | extra nuclear part |
| Proton | Positive 1.6 x 10-19C |
1.672 x 10-27 kg (1u) | Nucleus |
| Neutron | No charge | 1.672 x 10-27 kg (1u) | nucleus |
What are the limitations of J.J. Thomson’s model of atom?
According to J.J Thomson's model of an atom, an atom consists of a positively charged sphere with electrons embedded in it. However, it was later found by the Rutherford that the positively charged particles reside at the centre of atom called the nucleus, and the electron revolve around the nucleus.
What are the limitations of Rutherford model of the atom?
According to Rutherford model, there is a positively charged centre in an atom called the nucleus and the electron present in nucleus revolve aroun it, in well- defined orbits. But, since an electron is charged particle. Thus, while revolving around the nucleus it lose energy and finally fall into the nucleus. If this were so, the atom should be highly unstable and hence matter would not exist in the form that we know.
Describe Bohr's model of the atom ?
According to Bohr's theory:
(i) The atom consists of a small (positively charged) nucleus at its centre.
(ii) The whole mass of the atom is concentrated in the nucleus and the volume of the nucleus is smaller than the volume of the atom by a ratio of about 1 : 105.
(iii) All the protons and neutrons of the atom are contained in the nucleus.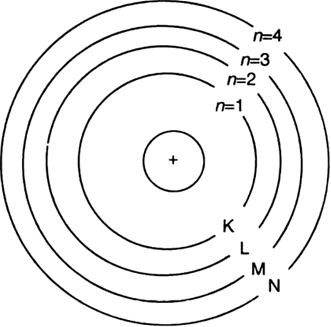
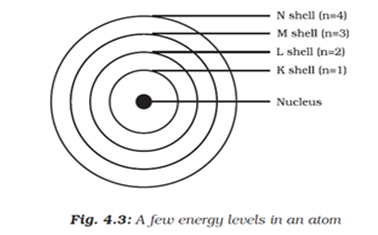
(iv) The electrons of the atom revolve around the nucleus in definite circular paths known as orbits or which are designated as K, L, M, N etc. or numbered as (n) = 1, 2, 3, 4 etc. outward from the nucleus.
(v) Each orbit is associated with a fixed amount of energy. Therefore, these orbits are also known as energy levels or energy shells.
(vi) The energy of the atom changes when an electron jumps from one state (energy level) to another state (energy level). As long as an electron remains in a particular orbit, it does not lose or gain energy.
Compare all the proposed models of an atom given in this chapter.
1).Thomson proposed the model of an atom to be similar to that of a watermelon. In which the positive charge in atom is spread all over like the red edible part of the watermelon, while the electrons are studded in the positively charged sphere, like the seeds in the watermelon.
On the base of this model; Thomson proposed that:
i)An atom consists of a positively charged sphere and the electrons are embedded in it.
ii)The negative and positive charges are equal in magnitude. So, the atom as a whole is electrically neutral.
2.)Rutherford designed a model in which fast moving alpha (a)-particles were made to fall on a thin gold foil. On the basis of above experiment Rutherford concluded that:
i)Most of the space inside the atom is empty because most of the (a)-particles passed through the gold foil without getting deflected.
ii)Very few particles were deflected from their path, indicating that the positive charge of the atom occupies very little space.
iii)A very small fraction of (a)-particles were deflected by 1800, indicating that all the positive charge and mass of the gold atom were concentrated in a very small volume with in the atom.
3.)Neils Bohr. Since charged bodies moving in circular motion emit radiations. This will lead to loss of energy of the moving electron and ultimately giving unstable model of atom. To explain stability of atom and atomic spectra, Bohr suggested that electrons are moving round the nucleus in orbits which have fixed energy shells. There is a loss or gain in energy of electron when it moves from one orbit to the other.
Summarize the rules for writing of distribution of electrons in various shells for the first eighteen elements.
Following rules are followed to fill electrons in different energy levels,
(i) If n gives the number of orbit or energy level, then 2n2 gives the maximum number of electrons possible in a given orbit or energy level. Thus
First orbit or K-shell will have 2n2 =2 x 1 = 2 electrons
Second orbit or L-shell will have 2n2 = 2 x 22 =8 electrons
Third orbit or M-shell will have 2n2 = 2 x 23 =18 electrons
(ii) If it is the outermost orbit, then it should have not more than 8 electrons.
(iii) There should be stepwise filling of electrons in different orbits, i.e., electrons are not accommodated in a given orbit if the earlier orbits or shells are incompletely filled.
Define valency by taking examples of silicon and oxygen.
The combining capacity of an element is called its valency. The number of electrons present in the outermost shell of an atom gives its valency. But, if the number of valence electrons of the atom of an element is less than or equal to four, then the valency of that element is equal to the number of valence electrons. For example, the atom of silicon has four valence electrons. Thus, the valency of silicon is four.
On the other hand, if the number of valence electrons of the atom of an element is greater than four, then the valency of that element is obtained by subtracting the number of valence electrons from eight. For example, the atom of oxygen has six valence electrons. Thus, the valency of oxygen is (8 − 6) i.e., two.
Explain with examples, (i) Atomic number, (ii) Mass number, (iii) Isotopes, and (iv) Isobars. Give any two uses of isotopes also.
(i) Atomic number is defined as number of protons present in the nucleus of an atom. For example, there are 6 protons in carbon, so the atomic number of carbon is 6. All atoms are characterized by their atomic numbers.
(ii) Mass number is defined as the sum of the total number of protons and neutrons present in the nucleus of an atom. For example there are 6 protons and 6 neutrons in the nucleus of carbon, so its mass number is 12.
(iii) Isotopes are atoms of the same element thus having same atomic number but different mass number. For example, chlorine has two isotopes with atomic number 17 but mass numbers as 35 and 37.
(iv) Isobars are such atoms which have same mass number but different atomic numbers. Thus isobars are different elements. For example, Ne has atomic number as 10 and sodium has atomic number as 11 but both of these have mass numbers as 22.
Uses of Isotopes
(i) Isotope of cobalt, ( Co), is used in the treatment of cancer.
(ii) Isotope of uranium (235U) is used as a fuel in nuclear reactors.Na+ has completely filled K and L-shells. Explain.
An atom of Na has a total of 11 electrons. Its electronic configuration is 2, 8, 1. But, Na+ ion has one electron less than Na atom i.e., it has 10 electrons. Therefore, K-shell contains 2 electrons and L-shell 8 electrons. Thus, Na+ has completely filled K and L-shells.
If bromine atom is available in the form of, say, two isotopes (49.7%) and (50.3%), calculate the average atomic mass of bromine atom.
Isotope of bromine with atomic mass 79 u = 49.7%
So, Contribution of 79 Br to atomic mass of bromine =
Isotope of bromine with atomic mass 81 u = 50.3%
therefore, Contribution of 81 Br to atomic mass of bromine =
Hence, atomic mass of bromine atom = 39.26 + 40.74 = 80.0 u
If Z = 3, what would be the valency of the element? Also, name the element.
If z = 3, i.e., atomic number is 3. Its electronic configuration is 2, 1. Hence, the valency of the element is 1 (since the outermost shell has only one electron). Then element is lithium. It has distribution of electrons as 2, 1.
Composition of the nuclei of two atomic species X and Y are given as under
X Y
Protons = 6 6
Neutrons= 6 6
Give the mass numbers of X and Y. What is the relation between the two species?
Mass number of X = 6+6 = 12
Mass number of Y = 6+8 = 14
Since X and Y both have atomic numbers as 6 but mass numbers are different, therefore, these are isotopes to each other.
For the following statements, write T for True and F for False.
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
(b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
(c) The mass of an electron is about 1/2000 times that of proton.
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine.
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons. (F)
(b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral. (F)
(c) The mass of an electron is about 1/2000 times that of proton. (T)
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine. (T)
Rutherford’s α-particle scattering experiment was responsible for the discovery of
A.
Atomic nucleus
B.
Electron
C.
Proton
D.
Neutron
B. FALSE
C. FALSE
D. FALSE
Isotope of an element has
A.
Same physical properties
B.
Different chemical properties
C.
Different number of neutrons
D.
Different atomic numbers.
B. TRUE
C. FALSE
D. FALSE
Sponsor Area
Complete the following Table:
|
Atomic number |
Mass number |
Number of neutrons |
Number of protons |
Number of electrons |
Name of the atomic species |
|
9 |
- |
10 |
- |
- |
- |
|
16 |
32 |
- |
- |
- |
Sulphur |
|
- |
24 |
- |
12 |
- |
- |
|
- |
2 |
- |
1 |
- |
- |
|
- |
1 |
- |
1 |
0 |
- |
|
Atomic number |
Mass number |
Number of neutrons |
Number of protons |
Number of electrons |
Name of the atomic species |
|
9 |
19 |
10 |
9 |
9 |
Fluorine |
|
16 |
32 |
16 |
16 |
16 |
Sulphur |
|
12 |
24 |
12 |
12 |
12 |
Magnesium |
|
1 |
2 |
1 |
1 |
1 |
Deuterium |
|
1 |
1 |
0 |
1 |
0 |
Protium |
How do you establish that matter is electrical in nature?
Matter is made of atom.The protons and electrons create attraction and repulsion forces as these atomic particles have an electric charge. Thus, it indicate that matter is electrical in nature. For example,
(i) When an ebonite rod is rubbed with a cat skin, ebonite rod gets negatively charged and cat skin positively charged.
(ii) A glass rod on rubbing with a silk cloth gets a positive charge.
(iii) A nylon comb rubbed with hair attracts bits of paper to it.
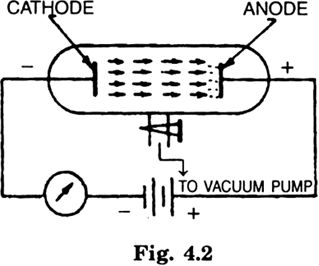
What is a discharge tube?
The discharge tube is a glass tube which is 70 cm long and having a diameter of 5 cm. Two metal electrodes are sealed at the two ends, one of which is connected to the negative terminal of a battery and the other to the positive terminal and a gas at low pressure inside the tube. A side tube is fused at the centre of the glass tube which serves to pump out air from it, using a suction pump.
What are the cathode rays? How are they produced?
When the pressure in the discharge tube (Fig) falls below 0.001 mm Hg and a potential difference of about 10,000 volts is applied across the electrodes, the walls of the discharge tube opposite to the cathode starts glowing with a faint greenish light. This is due to the bombardment of walls by some rays emerging from the cathode or negative electrode. These rays are known as cathode rays. These observations lead to the conclusion that cathode rays consist of rapidly moving negatively charged particles. These are called electrons and are shot out from the cathode of a discharge tube when an electric current is passed at high voltage through a gas at very low pressure.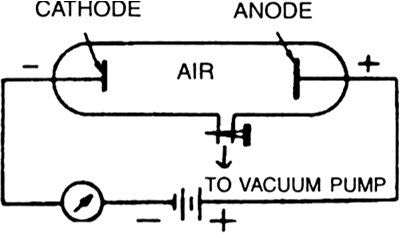
What are important properties of cathode rays?
Or
Give four characteristics of cathode rays.
Important properties of cathode rays:
(i) Cathode rays travel in straight lines and thus cast shadows of objects placed in their path.
(ii) Cathode rays possess material particles because they can rotate a light paddle wheel placed in their path.
(iii) They are deflected towards positive plate thus showing that these are negatively charged particles known as electrons.
(iv) They ionise gas through which they pass.
(v) They are deflected by magnetic fields.
(vi) The nature of cathode rays is independent of the material of cathode. Hence they are common constituents of all matter.
(vii) They can penetrate through thin metallic sheet.
(viii)They can produce X-rays.
(ix) The mass of a cathode ray particle is very-very small as compared to the mass of the atom from which it is formed.
State the observations made, while conducting a discharge experiment, which showed that
(i) Cathode rays travel in straight lines.
(ii) Cathode rays are made up of material particles having mass and kinetic energy.
(iii) Cathode rays are negatively charged.
(i) Cathode rays cast shadow of the objects placed in their path. This observation shows that cathode rays travel in straight lines.
(ii) It was observed that cathode rays could rotate the paddle of light wheel. This suggested that cathode rays possess mass and hence kinetic energy.
(iii) The cathode rays were deflected towards positive plate of the electric field applied in their path. This shows that cathode rays are negatively charged particles because only opposite charge attract each other.
How is the flow of current in a cathode ray experiment explained knowing that gases are bad conductors of electricity?
The gas in the discharge tube experiment (at low pressure and high voltage) gets decomposed. Negatively charged particles are produced which travel from cathode to anode, thus facilitating the flow of current. Under other conditions, e.g., 1-atmosphere pressure, no dissociation of gas occurs and no current flows.
Why does a part of the glass glow in the discharge tube experiment ? Name the scientist who first performed the discharge tube experiment.
Cathode rays are streams of electrons observed in vacuum tubes. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, the glass opposite of the negative electrode is observed to glow, due to electrons emitted from and travelling perpendicular to the cathode.William Crooks in 1879 first performed this experiment.
What are X-rays?
X-rays are electro-magnetic waves with very short wavelengths. These are formed when fast moving electrons are suddenly stopped by putting some obstructions like a block of tungsten metal. For example, in the discharge tube, X-rays can be produced if the electrons/ cathode rays are made to strike against the metal target.
The important feature of X rays is that it can penetrate or pass through the human body and produce shadow-like images of structures such as bones, some of the organs, and signs of disease and injury.
Give the properties and use of X-rays.
Properties of X-rays:
(i) X-rays travel in straight lines with speed of light.
(ii) X-rays are not deflected by electrical and magnetic fields.
(iii) They are more penetrating than cathode rays.
(iv) They can pass through opaque materials such as black paper and affect a photographic plate wrapped in it.
(v) They cause fluorescence in several materials. A plate coated with barium platinocyanide, zinc sulphide etc. becomes luminous when exposed to X-rays.
Uses of X-rays :
(i) They are useful to locate the broken parts of the body such as bones because solid bone stops X-rays from passing through it and are seen as an opaque image in the X-rays film.
(ii) They are used by detective departments to examine the contents of a parcel without opening it and to detect diamonds/gems concealed by smugglers within their body.
(iii) They are used in the treatment of tumours and cancer in the animals and the human beings.
(iv) They are quite helpful in the study of the nature and structure of the materials.
Describe how X-rays are used to locate cracks in fractured bones.
X-rays are used to locate cracks in the fractured bones because these can penetrate the flesh easily but are stopped by the bones which are quite hard. When X-rays are focussed on the fractured part of the body, then a clear image of the bones in that part of the body is formed on a photographic film placed behind the body part. The cracks in the bones can be seen clearly in the X-ray photograph.
Give one use of X-rays stating the property which makes this use possible.
X-rays are used to locate cracks in the fractured bones. This is because X-rays can penetrate the flesh easily but are stopped by the bones. Thus, when X-rays are focussed on the body, a clear image of the bones is formed on the photographic plate placed behind the body. A study of the photographic plate can easily reveal the cracks, if any, in the bones.
Give the mass and charge of an electron.
(i) The mass of an electron is about 1/1840 that of an hydrogen atom, i.e., about 9.0 x 10-31 kg.
(ii) An electron is negatively charged particle and has a charge of 1.6 x 10-19 coulomb. This is one unit of charge.
Comment on the statement: “Electrons are common constituents of all matter”.
The statement is true because :
(i) Whatever be the nature of the gas or the material of the cathode, the electrons have the same charge to mass (e/ m) ratio i.e., 1.7589 x 1011 coulomb per kg.
(ii) The electrons obtained by different methods are identical.
What were the observations that led to the conclusion that cathode rays are negatively charged particles?
When an electric discharge at a very high voltage was passed through discharged tube containing air at a very low pressure, it was observed that cathode rays emanated from cathode and proceeded towards positive plate. This led to the conclusion that cathode rays are negatively charged particles.
Describe an experiment to show that cathode rays travel in straight lines.
William Crookes in 1879 demonstrated that cathode rays travel in straight lines. He placed an opaque object like metal cross in the path of cathode rays in a discharge tube. It was observed that a sharp shadow of the cross was cast on the glass behind the cross. A shadow of metal cross under the circumstances can be formed only if cathode rays travel in straight lines and cannot bend round the corners of the metal cross.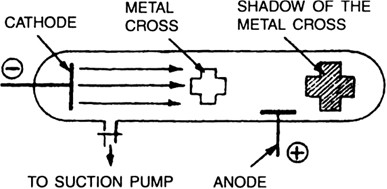
What is phosphorescence?
Phosphorescence is luminescence that occurs when energy is supplied by electromagnetic radiation, such as ultraviolet light. The energy source excite an atom from a lower energy state into an excited higher energy state; then the electron releases the energy in the form of light when it falls back to a lower energy state. The light emission continues even after the radiation, responsible for producing phosphorescence is removed.
What are the different stages observed when a discharge tube is connected to a high voltage source and the pressure of the air in it is reduced gradually?
The following observations are made in the discharge tube under high voltage when the air pressure is gradually reduced.
(i) At normal air pressure — No change
(ii) At slightly reduced air pressure — Unsteady light emission
(iii) At still lower pressure — Tube fills up with uniform glow with magneta-red colour
(iv) At pressure below 0.001 mm Hg — Tube appears dark and the cathode starts glowing with a faint greenish light.
What happens when the cathode rays are passed through an electric field between two parallel plates ? Can one determine the nature of charge of the particles constituting the cathode rays from this experiment ? If so how?
When the cathode rays are passed through an electric field between two parallel plates, cathode rays are deflected towards positive plate. This determines the charge of the particles constituting the cathode rays. As these are deflected towards positive plates, the particles of cathode rays are negatively charged.
A student weighs 30 kg. Suppose his entire body is made up of electrons. How many electrons are there in his body? Compare the total number of electrons in his body with the population of India.
Mass of electron = 9.1 x 10-31kg.
Number of electron in the body of student= total mass/ mass of each electrons=30kg/9.1 x 10-31 kg
Therefore,
The student is made up of approximately 3.29 x1031 electrons.
Population of India is about 100 crores
3.29 x 1031/109 =3.29 x 1022
Therefore,
Number of electrons in the body of the student is 3.29 x 1022 times the population of India.
What are anode or positive rays? How are they produced?
E. Goldstein found that in the discharged tube if the cathode used is perforated and high voltage is applied between the electrodes when the pressure within the tube is below 0.001 mm Hg (Fig. 4.4), it will be observed that new type of rays come through this experiment.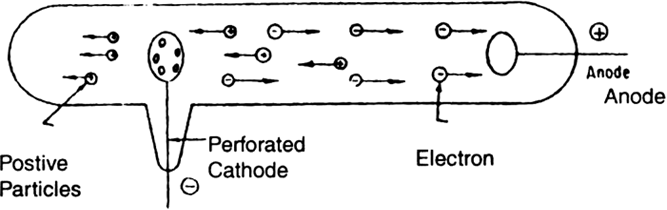
Fig. Production of positive rays in a discharge
perforations in the cathode. Since these rays come through perforations in the cathode, these were initially called canal rays. These are also called positive or anode rays, as they move away from the anode side through the gas. In a discharge tube, when the gas atoms lose electrons, they acquire a positive charge and thus move away from the anode. Thus, anode rays are positively charged particles which are emanated from the anode of a discharge tube when a current of high density is passed through a gas at very low pressure. The mass and charge of the anode rays depends on the nature of the gas taken in the discharge tube.
Sponsor Area
What are the important properties of anode rays?
These are Properties of anode rays as under:
(i) They consist of Positively charged particles. Their mass is virtually the same as that of the atoms from which they are derived and is found to be equal to the atomic mass of the gas in the discharge tube.
(ii)They travel in straight lines.
(iii)They are capable of producing physical and chemical changes.
(iv) They can penetrate thin metal foils.
(v)They can produce ionization in gases.
(vi)They are deflected by electrical and magnetic fields just as the cathode rays but in opposite directions showing that they are oppositely charged, i.e., they carry positive charge. Since their deflection is very little, they consist of very heavy particles.
What observation led to the conclusion that the nature of the anode rays depend upon the gas used in the cathode rays tube?
It was found that e/ m value for positive rays are different when the enclosed gas in the cathode rays tube is different. This is possible when different gases give different types of positive rays which contain particles having different masses and different charges. Thus, the mass and charge of positive ray particles depends upon the gas which is taken in the discharge tube. The positive particles obtained from hydrogen gas are the lightest and have the highest charge to mass ratio.
Can you predict the mass of the particles of anode rays from the nature of the gas used hydrogen?
In the case of hydrogen gas the anode rays consist of proton. A proton possesses one unit of positive charge and one unit of mass.
Explain the conduction of electricity through gases at very low pressures.
When we apply high electric voltage, the electric energy splits the gasesous molecules into positively charged particles and negatively charged particles. The negatively charged particles form cathode rays and positively charged particles form anode rays. These charged particles can conduct electric current through the discharge tube. However, if the gas in the discharge tube is at the atmospheric pressure, the large number of gas atoms collide with the electrons and prevent them from reaching the anode. In such circumstances no current flows through the discharge tube. When the gas pressure is very low, there are a few gas atoms in the discharge tube. In this situation the movement of electrons towards the anode is not hindered and thus the gas conducts electricity.
What is a proton ? What are its characteristics?
A proton is the lightest positive particle. It is obtained when the gas inside the cathode rays tube is hydrogen.
Characteristics of Proton :
(i) A proton is one of the fundamental particles of the atom and is present in the nucleus of all atoms.
(ii) Charge: The charge on a proton is equal in magnitude but opposite in sign to that of an electron. Thus, it possesses a unit positive charge or + 1.6 x 10-19 coulombs.
(iii) The mass of a proton is equal to that of a hydrogen atom, i.e., 1.672 x 10-27 kg which is about 1837 times the mass of the electron.
In what respects cathode rays differ from positive or anode rays?
Or
What are cathode rays and positive rays?
Cathode rays are made up of negatively charged particles called electrons. The nature of cathode rays does not depend on the nature of the gas from which these are produced. The mass of a cathode rays particle is very very small compared to the mass of the atom from which it is formed. These particles were found to be about 2000 times smaller in mass than the hydrogen atom.
Anode rays are a stream of positively charged particles. The mass of an anode rays particle is equal to the mass of the atom from which it is formed. The nature of the anode rays depends on the gas from which these are produced.
Comment:All substances contain protons in addition to electrons.
There are numerous experiments to show that protons are present along with electrons in all substances. Protons are emitted during radioactive decay of various radioactive substances. Nitrogen present in the upper layer of atmosphere is converted into radioactive carbon by emission of a proton when the cosmic rays coming from the sun fall on it.
![]()
It is thus concluded that protons like electrons are the constituent particles of all matter.
What is the nature of charge on different rays:
(i) X-rays
(ii) cathode rays
(iii) gamma rays
(iv) anode rays?
The nature of charge ion on the rays are:
(i) X-rays—no charge
(ii) Cathode rays—negative charge
(iii) Gamma rays—no charge
(iv) Anode rays—positive charge.
What are the differences in the discharge tubes used to study cathode rays and the positive rays?
A cathode ray discharge tube is essentially a glass tube with closed ends. Two metal plates which serve as cathode and anode are sealed at the two ends of the glass tube.
The discharge tube used to study anode rays has the perforated plate serving as cathode and is sealed in the middle of the glass tube.
What are canal rays?
The Canal rays are positively charged radiations consisting of particles which have a charge equal in magnitude but opposite in sign to that of the electron. The mass of a canal ray particle is 2000 times as that of the electron. This particle is known as proton.
How were the results of discharge tube experiments interpreted by J. J. Thomson in terms of model of an atom?
The identification of cathode rays constituting charged electrons led to the conclusion that
(i) Electrons are present in an atom.
(ii) These electrons are embedded in a sphere of positive charge.
(iii) The comparison of e/m values for electron and H+, Na+ or other ions showed that the remaining part should be 2000 times heavier than the electron.
On this basis J.J. Thomson gave a model of the atom in which electrons and mass were distributed throughout the full size of atom. The size of the atom was estimated to be about 10-10 m. fig. gives model of three atoms containing one, two and three electrons. This model was considered to be very unstable.
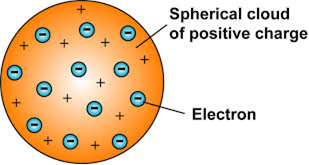
Thomson proposed the model of an atom to be similar to that of watermelon. the positive charge in the atom is spread all over like the red edible part of the watermelon, while the electrons are studded in the positively charged sphere, like the seeds in the watermelon.
Give an evidence for the existence of nucleus in an atom.
Or
What important informations are furnished about the nucleus of an atom by α-particle scattering experiment of Rutherford?
When Rutherford bombarded thin sheet of gold foil with α -particles (α-particles are doubly charged helium ions, He2+), he found that:
(i) Most of the α-particles passes through the foil without any deflection. He calculated that only one particle in 105 bounced back. This shows that most of the space inside the atom is empty and hollow.
(ii) Some of the α-particles were deflected through various angles while a very small number were actually deflected by as much as 180°.
This shows that:
(a) There is a heavy positively charged centre inside the atom. This centre is known as nucleus.
(b) Since only a very small fraction of α-particles were deflected through large angles, the nucleus is situated in a very small volume of the atom and is positively charged. The nucleus was found to be about 105 times smaller than the total area occupied by the atom as a whole.
(c) Since α-particles deflected by the nucleus have an appreciable mass, it means that the entire mass of the atom lies inside the nucleus.
Who discovered neutrons? What observation lead to the discovery of neutrons?
Chadwick discovered neutrons. The observation that the atomic masses of elements other than hydrogen could not be explained on the basis of the presence of only protons and electrons in an atom, and the atomic masses of all elements are much higher than that expected on the basis of the presence of only protons and electrons in an atom led to the discovery of neutron.
What is a nucleus?
The small positively charged central part of an atom is called nucleus. All the protons and neutrons are present in this region.
What determines the number of positive charges on the nucleus?
The Number of protons an atom contains determines the number of positive charges on the nucleus.
In Rutherford's experiment, some of the α-particles when bombarded against a gold leaf, were repelled. Give the reasons for this observation.
Or
Give experimental evidence to show that the nucleus of an atom is positively charged.
α-particles are positively charged helium ions containing two protons and two neutrons each. In Rutherford's experiment only those α-particles, which bombarded the central part i.e., positively charged nucleus, were repelled. As same charges repel each other, therefore, it was concluded that the nucleus of an atom is positively charged.
Why is an atom neutral in spite of the presence of charged particles in it?
The number of negatively charged electrons (total negative charge) is the same as the number of positively charged protons (total positive charge) in the atom of an element.
How was it shown that an atom has a lot of empty space within it?
When α-particles are allowed to strike a very thin gold foil, it is found that most of these particles pass through the foil without any deflection. It is observe that one particle in 12000 bounces back.
Thus, Rutherford calculated that the radius of the nucleus is about 105 time less than the radius of the atom. hence, atom has a lot of empty space within it.
How was it shown that atomic nuclei are positively charged?
Or
Describe an experiment to show that the nucleus of an atom contains positively charged particles.
The fact that atomic nuclei are positively charged can be shown by performing Rutherford’s α-particle scattering experiment. Take a thin sheet of metal foil. Allow α-particles to bombard over it. It will be observed that only a small fraction of α-particles (positively charged) are deflected through large angles and the rest pass through the foil without any deflection. This shows that positive charge of the atom is concentrated at the centre or the nucleus.
What observations led Rutherford to believe that central part of the atom is positively charged?
Rutherford designed a model in which fast moving alpha (a)-particles were made to fall on a thin gold foil. On the basis of above experiment Rutherford concluded that:
i) Most of the space inside the atom is empty because most of the (a)-particles passed through the gold foil without getting deflected.
ii) Very few particles were deflected from their path, indicating that the positive charge of the atom occupies very little space.
iii) A very small fraction of (a)-particles were deflected by 1800, indicating that all the positive charge and mass of the gold atom were concentrated in a very small volume with in the atom.
Describe the magnitude of the size of an atom in comparison to its nuclear size.
The radius of an atom is 105 times greater than the radius of the nucleus.
![]()
What are the characteristic features of the Thomson model of atom?
Characteristic features of Thomson model of an atom are:
(i) An atom consists of a positively charged sphere and the electrons are embedded in it.
(ii) The negative and positive charges are equal in magnitude. So, the atom as a whole is electrically neutral.
Describe the essential properties of the atomic nucleus. Compare these with the properties of the electron.
The nucleus is a heavily charged positive centre located in a very small space of around 10-15 m. An electron is a very small negatively charged particle with well established charge to mass ratio. The charge on electron forms the smallest unit of charge on atomic particles.
What was the model of atom as proposed by Rutherford?
The main features of Rutherford's model of an atom are:
(i) The atom consists of a positively charged centre called the nucleus.
(ii) Most of the mass is concentrated in the nucleus.
(iii) The volume of the nucleus is very small compared to the total volume of the atom.
(iv) The nucleus is surrounded by the negatively charged electrons which are revolving round the nucleus at very high speeds like the planets revolving round the sun.
What was the main objection to Rutherford’s model of atom?
Rutherford proposed that the electrons revolve around the nucleus in well- defined orbits.But, electron is charged particle any charge particles during acceration radiate energy circular orbit would undergo acceleration. Thus, the revolving electron would lose energy and finally fall into the nucleus. If this were so, the atom should be highly unstable and hence matter would not exist in the form that we know.
Describe the nuclear (Bohr’s) model of an atom.
According to Bohr’s theory:
(i) The atom-consists of a small (positively charged) nucleus at its centre.
(ii) The whole mass of the atom is concentrated at the nucleus and the volume of nucleus is smaller than the volume of the atom by a ratio of about 1 : 105.
(iii) All the protons and neutrons of the atom are contained in the nucleus.
(iv) The electrons of the atom revolve round the nucleus in definite circular paths known as orbits or shells which are designated as K, L, M, N etc. or numbered as (n) = 1, 2, 3, 4 etc. outward from the nucleus.
(v) Each orbit is associated with a fixed amount of energy. Therefore, these orbits are also known as energy levels or energy shells.
(vi) The energy of the atom changes when an electron jumps from one state (energy level) to another state (energy level). As long as an electron remains in a particular orbit, it does not lose or gain energy.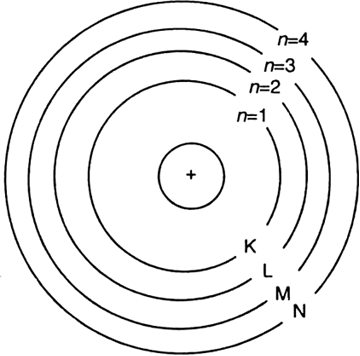
In Rutherford experiment of scattering of alpha particles, can we take foil of any other metal instead of gold?
In Rutherford experiment of scattering of alpha particles, we can take foil of any other metal if it is highly malleable because for this experiment we need a very thin foil. If the observation is taken by heavy metal like gold or Platinium, silver than the observation would be same but if it would be taken by light metal like lithium than the massive alpha particles will push the nucleus and may not be deflected back.
When an electron jumps from energy level K to energy level L, why does the energy of the atom increase?
An electron revolving in an orbit (energy level) such as K, has a fixed amount of energy. When it jumps from K to L, it acquires energy of level L which is higher and thus the electron acquires more energy than it previously had. This leads to the overall increase in the energy of the atom.
Why are the shells in which the electrons revolve around the nucleus of an atom called energy levels?
An electron revolving in a cell is associated with a definite amount of energy. Its energy changes when it jumps to another cell, that is to say when it goes from one level of energy to another level of energy. Thus, a shell also gives the energy of an electron besides its location and are, therefore, called energy cells or levels.
How does Bohr’s model of atom explain characteristic spectra of different atoms and ionisation of gases in the discharge tube experiment?
In Bohr's model of atom, the electrons can occupy orbits with discrete energy levels only. Thus, when an electron falls from a higher energy level to a lower one, the difference in energy is radiated in the form of electromagnetic radiation of a fixed wavelength only. Since each atom has its specific energy levels, it can emit radiations of specific wavelength. This explains why different atoms give different or characteristic atomic spectra.
When the electron is so excited due to external energy that it is able to overcome force between it and the positively charged nucleus, it comes out of the atom. This explains the formation of cathode rays in the discharge tube. This is also known as ionization of gases.
What is an orbit?
Orbit is the path of the electron around the nucleus. At which electron revolve around the nucleus.
Describe the essential features of the model of atom proposed by E. Rutherford. How is it different from that proposed by J.J. Thomson?
E. Rutherford proposed that each atom consists of a positive nucleus around which negatively charged electrons are revolving round the nucleus just like the planets move round the sun in fixed orbits in our solar system. According to this model most of the mass is concentrated in a small central part of the atom whereas J.J. Thomson proposed that , an atom consists of a positively charged sphere and the electrons are embedded in it. He also proposed that atom is electrically neutral.
What are the fundamental contributions of E. Rutherford in understanding the structure of atom?
Fundamental contributions of E. Rutherford in understanding the structure of the atom are:
(i) There is a positively charged centre in an atom called the nucleus. Nearly all the mass of an atom resides in the nucleus.
(ii) Most of the mass is concentrated at the nucleus. The electrons revolve around the nucleus in circular paths.
(iii) The size of nucleus is 105 times smaller than the atom.
What was the concept incorporated by Neils Bohr in the model of atom proposed by him?
He gave a new concept that particles at atomic level would behave differently from the macroscopic objects. He suggested that electrons could revolve in stable orbits without continuously radiating or losing energy. According to Bohr's model, an electron revolves in an orbit with well-defined orbit.
What is a neutron? What are its characteristics?
Neutrons are present in the nucleus of an atom.
Charge: It is a neutral particle because it has no charge.
Mass of neutron: Mass of neutron is 1.0086654 a.m.u. or 1.6749 x 10-27 kg.
Compare an electron, a proton and a neutron in respect of their symbol, mass and charge.
|
Particle |
Symbol |
Mass |
Charge |
|
Electron |
0e-1
|
9 X 10-31 kg (1/1840 of H |
Charge of electron is 1.6 x 10-19 coulombs unit.
|
|
Proton |
1p+1
|
1.67 x 10-27 kg or 1 u
|
positive or + 1.6 x 10-19 coulombs
|
|
Neutron |
1n0 |
1 u or 1.6749 x 10-27 kg. |
No charge |
State the fundamental particles present in an atom of any element.
The fundamental particles in an atom of any element are:
(i) Electron (0e-)
(ii) Proton (1p+1)
(iii) Neutron (1e0).
State the similarities and dissimilarities between protons and neutrons.
Similarities of protons and neutrons:
(i) Both protons and neutrons are present in the nucleus of an atom.
(ii) The mass of a neutron is approximately equal to the mass of a proton, i.e., 1.67 x 10-27 kg.
Dissimilarities between protons and neutrons:
Protons possess a unit positive charge (1.6 x 10-19 coulomb).
Neutron carries no charge, i.e., it is electrically neutral particle.
What will the addition of a neutron to the nucleus of an atom do?
The addition of a neutron to the nucleus of an atom, will increase the atomic mass of the atom.
Which constituent particles of the atom determine the following:
(i) Mass of the atom.
(ii) Size of the atom,
(iii) Charge on the nucleus.
(i) Protons and neutrons.
(ii) Electrons
(iii) Protons.
Sponsor Area
Can you explain why scattering experiment cannot be used to prove the existence of neutrons?
We cannot use scattering experiment to prove the existance of neutrons because the neutrons are neutral particles and so there will be no repulsion between positively charged α-particles and neutrons.
Nucleus does not contain any electrons. Even then the β-particle emission has been described as the ejection of an electron from the nucleus. Comment.
It is believed that electrons are produced as a result of decay of neutrons as indicated below:
![]()
The electron produced escapes as a (β-particle leaving the proton behind in the nucleus.
How does the emission of γ-rays affect the nucleus?
γ-rays are emitted as a result of rearrangement of neutrons and protons in a nucleus. Thus, the nucleus is not affected in terms of number of protons and neutrons due to emission of γ-rays.
On the basis of Thomson’s model of an atom, explain how the atom is neutral as a whole?
According to Thomson's model of an atom, the negative and positive charges are equal in number and magnitude. therefore that the atom as a whole is electrically neutral.
On the basis of Rutherford's model of an atom, which sub-atomic particle is present in the nucleus of an atom.
Protons reside in the nucleus of an atom on the basis of Rutherford's model of an atom.
Draw a sketch of Bohr's model of an atom with three shells.
Bohr’s model with three shells.
What do you think would be the observation if the α-particle scattering experiment is carried out using a foil of a metal other than gold?
If α-particle scattering experiment is carried out using a foil of any metal as thin as gold foil used by Rutherford, there would be no change in observations if heavy metal like gold use for example Platinium, sliver. but if it would be taken by light metal like lithium than the massive alpha particles will push the nucleus and may not be deflected back.
Name the three sub-atomic particles of an atom.
The sub-atomic particle of atom are:
(i) electron (0e-1)
(ii) proton (1p+1)
(iii) neutron (1n0)
Helium atom has an atomic mass of 4 u and has two protons in its nucleus. How many neutrons it have?
The mass of an atom is given by the sum of the masses of protons and neutrons present in the nucleus.
Mass of atom =Protons +Neutrons
Since helium atom has a atomic mass of 4 u and it has two protons. Two protons contribute 2 u to atomic mass.
4u =2u +Neutrons
Neutrons =4-2 =2
Hence, it must contain 2 neutrons.
State the suggestions made by Bohr and Bury with regard to distribution of electrons in different energy levels in the atoms of elements.
Or
Explain the arrangement of electrons outside the nucleus.
(i) Electrons are revolving around the nucleus in different orbits or shells. These energy shells are represented by numbers 1, 2, 3, 4 or K, L, M, N.
(ii) The maximum number of electrons in any shell cannot exceed 2n2 , where n is the number of that energy level. Thus for
K-shell, n = 1, no. of electrons = 2x12 =2
L-shell, n = 2, no. of electrons = 2x22 =8
M-shell, n = 3, no. of electrons = 2x32 =18
N-shell, n = 4, no. of electrons = 2x42 = 32.
(iii) The outermost orbit of an atom cannot have more than 8 electrons and the next to the outermost shell (penultimate shell) can have at the most 18 electrons.
(iv) It is not necessary that an orbit has its full quota of electrons before starting to fill the next higher orbit.
What is meant by electronic configuration of elements?
The systematic distribution of electrons in various orbits or energy shells of an atom is called the electronic configuration of elements.
If both K and L-shells of an atom are full, what is the total number of electrons contained in them?
Since the both of K and L- shell of an atom are full thus, the total number of electron present in atom is 10 (K = 2, L = 8).
Can electrons be arranged in second shell or L-shell without filling first shell or K-shell?
No, electron cannot be arranged in second shell without filling first shell because electrons first fill in lower value of energy and the energy of first shell is lower than second shell. So electrons will be arranged first in the K shell.
Fluorine atom has 9 electrons and 9 protons. How many energy shells it has?
Fluorine has 9 electrons, 2 electrons can be accommodated in the first shell, the remaining seven electrons are accommodated in the second shell or L-shell which has a maximum capacity of 8 electrons. So, fluorine can have two energy shells.
Magnesium atoms has 12 electrons. Which energy shell is incomplete?
There are 12 electrons in an atom of magnesium. K-shell contains 2 electrons and L-shell contains 8 electrons. Thus K and L-shell are full. Remaining 2 electrons (12-2-8 = 2) are accommodated in third or M-shell. M-shell can accommodate 18 electrons. So M-shell is incomplete.
Argon atoms has 18 electrons. How many energy shells or orbits are incomplete ? How many energy shell it contains?
Distribution of 18 electrons in argon atom is as follows:
K-shell = 2 electrons
L-shell = 8 electrons
M-shell = 8 electrons
K-shell and L-shell have maximum capacity of 2 and 8 electrons respectively. M-shell has a capacity of 18 electrons. Since, it is the outermost orbit and there is a rule that ‘the maximum number of electrons that can be accommodated in the outermost orbit is 8’. In the present case, M-shell is also complete. Thus, in argon atom, no shell is incomplete. Argon atom has three energy shells.
What are the similarities in the electronic structure of the following sets of atoms:No. of electrons in each atom is given in brackets:
i) lithium (3), sodium (11) and potassium (19).
ii) helium (2), neon (10) and argon (18).
iii)beryllium (4), magnesium (12), and calcium (20).
i) Lithium, sodium and potassium all have one electron in outermost shell. .
ii) Helium, neon and argon all have eight electrons in outermost shell.
iii) Beryllium, magnesium and calcium all have two electrons in outermost shell.
Write the distribution of electrons in carbon and sodium atoms.
Carbon has 6 electrons. So its electronic configuration is:![]()
Sodium has 11 electrons. Its electronic configuration is:
K-shell - 2 electrons
L-shell - 8 electrons or 2, 8, 1
M-shell - 1 electron
If K and L shells of an atom are full then what would be the total number of electrons in the atom?
Distribution of electrons in shell is as follows:
K-shell = 2 electrons
L-shell = 8 electrons
If K-shell and L-shell are full then, the total number of electrons are 10.
What are valence electrons?
Give two examples to illustrate the answer.
The number of electrons present in the outermost shell of an atom are known as valence electrons.
Examples:
|
Element |
No. of Electrons |
Electronic Configuration |
Valence Electrons |
|
Boron |
5 |
K=2,L= 3 |
3 |
|
Oxygen |
8 |
K=2,L= 6 |
6 |
From the above table, in case of boron there is 3 electron present in outermost shell therefore valence electron for boron is 3. In the same manner, oxygen have 6 electron in its outer most shell, hence it have 6 valence electron.
Which electrons of an atom decide the chemical properties of the element?
Valence electrons of an atom decide the chemical properties of the element.
How do you define valency of an atom?
The number of electrons determining the combining capacity of an atom is known as its valency.
For example, oxygen has six valence electrons but to combine with other atoms it must accept two electrons. So its valency is -2. The valency of lithium is one as these lose one electron, when combine with other atoms.
i) If the atom contains 1, 2 or 3 electrons in its outermost shell, then valency is equal to the valence electrons.
ii) If the number of electrons in the outermost shell of an atom is more than four then the valency is equal 8 minus number of valence electron present in outer most shell.
An element has 16 protons. How many electrons will be present in K, L and M shell of its atom. What will its electrovalency be?
No. of electrons = no. of protons = 16
Distribution of 16 electrons in atom is as follows:
K-shell = 2 electrons
L-shell = 8 electrons
M-shell = 6 electrons
Valence electrons in the element are 6.
∴
Valency or electrovalency is 8 - 6 = 2.
How will you find the valency of nitrogen, oxygen and fluorine:
K-Shell L-shell
Oxygen 2 6
Nitrogen 2 5
Fluorine 2 7
Valency of oxygen = 8 - 6 = 2
Valency of nitrogen = 8 - 5 = 3
Valency of fluorine = 8 - 7 = 1
Note : When the outermost shell of an atom contains 4 or more electrons, its valency is equal to 8 minus the number of electrons in the outermost shell.
What is atomic number? How has this concept improved the definition of an element?
(i) Atomic number of an element is equal to the number of protons present in the nucleus of its atom.
∴
Atomic number = Number of unit positive charges on the nucleus.
Since the atom as a whole is electrically neutral, therefore, atomic number is the same as the number of electrons around the nucleus of an atom.
(ii) Atomic number gives the position of the element in the periodic table. In terms of atomic number an element can now be defined as a substance comprising of atoms all of which have the same atomic number.
Why is atomic number is the fundamental property of elements?
All the elements are characterised by their atomic numbers. When elements react, their atoms either lose or gain electrons but their atomic number remains the same. Thus, atomic number is the fundamental property of the elements. Also, elements in periodic table are arranged in increasing atomic number.
Define the terms:
(i) atomic number
(ii) mass number, of an atom in an element.
(i) The atomic number (z) is the number of protons present in the nucleus of an atom.
(ii) Mass number (A) is sum of the number of protons (p) and the number of neutrons (n) in the nucleus of an atom.
Thus, A = n + p or = n + z. (z is atomic number.) For example the mass of carbon is 12 u because it has 6 protons and 6 neutrons, 6 u + 6 u = 12 u
Calculate the atomic number of an element whose atomic nucleus has mass number 23 and neutron number 12. What is the symbol of this element?
We have given the mass number of atom and Mass number is given by the formula,
Mass no. = atomic number + no. of neutrons
∴
atomic number = mass no. - no. of neutrons = 23 - 12 = 11
The element is sodium (Na).
The mass number of an element is 18. It contains 9 electrons. What is the no. of protons, neutrons? What is the atomic no. of the element
The mass number of the atom is given by the formula,
mass number = Total number of protons + Total number of neutrons.
(i) Number of protons = no. of electrons = 9
(ii) Number of neutrons = mass no. - no. of protons = 18 - 9 = 9
∴
(iii) Atomic number = no. of protons = 9.
The atomic number of electron is 9. Element is Fluorine.
Explain the method to write the electronic configuration of the atom of an element whose mass number (A) is 35 and atomic number 16. What are the no. of electrons, protons and neutrons?
Number of protons = atomic number = 16
∴ No. of neutrons = mass no. - no. of protons = 35 - 16 = 19
∴ No. of electrons = no. of protons = 16.
Writing of electronic configuration
Distribution of 16 electrons in argon atom is as follows:
K-shell = 2 electrons
L-shell = 8 electrons
M-shell = 6 electrons
K-shell and L-shell have maximum capacity of 2 and 8 electrons respectively. M-shell has a capacity of 18 electrons. Since, it is the outermost orbit and there is a rule that ‘the maximum number of electrons that can be accommodated in the outermost orbit is 8’. In the present case, M-shell has incomplete shell.
An element has an atomic number of 19 and atomic mass of 39.
(i) How many electrons are there in each atom of the element?
(ii) How are these electrons arranged in the atom?
Or
Write electronic distribution in an atom of potassium.
(i) 19 electrons = atomic number.
(ii) The electronic configuration of the given element is,
K = 2, L = 8, M = 8, N = 1.
Potassium with 19 electrons has the electronic configuration as K = 2, L = 8, M = 8, N = 1. Why cannot you think of 9 electrons in M-shell when it can contain 18 electrons (2n2 = 18)?
L-shell can contain 18 electrons but Bohr and Bury scheme also says that the outermost orbit in an element cannot contain more than 8 electrons. Therefore, in accordance with this scheme, the nineteenth electron finds a place in the next shell or in N-shell.
Write the electronic configuration of an element X, whose atomic number is 12.
No. of electrons = atomic no. = 12.
Thus, K-shell contains = 2 electrons
L-shell contains = 8 electrons
M-shell contains = 2 electrons
Thus, electronic configuration of X becomes 2, 8, 2.
If an element M has atomic weight 24 and atomic number 12, how many neutrons does its atom contain? How many electrons will be present in K, L and M energy shells of its atom?
Number of electron = number of protons =12
No. of neutrons = Atomic mass - No. of protons = 24 - 12 =12
Thus,
total number of neutrons =12
So,
Electronic configuration is
K - cell 2 electrons
L - cell 8 electrons
M - cell 2 electrons.
Give the nuclear composition and electronic configuration of 18 elements with atomic number 1 to 20.
|
Name of element |
Symbol |
Atomic number |
Number of protons |
Number of Neutrons |
Number of Electrons |
Distribution of Electrons |
Valency
|
||||
|
K |
L |
M N |
|
||||||||
|
Hydrogen |
H |
1 |
1 |
0 |
1 |
1 |
- |
- |
- |
1 |
|
|
Helium |
He |
2 |
2 |
2 |
2 |
2 |
- |
- |
- |
0 |
|
|
Lithium |
Li |
3 |
3 |
4 |
3 |
2 |
1 |
- |
- |
1 |
|
|
Beryllium |
Be |
4 |
4 |
5 |
4 |
2 |
2 |
- |
- |
2 |
|
|
Boron |
B |
5 |
5 |
6 |
5 |
2 |
3 |
- |
- |
3 |
|
|
Carbon |
C |
6 |
6 |
6 |
6 |
2 |
4 |
- |
- |
4 |
|
|
Nitrogen |
N |
7 |
7 |
7 |
7 |
2 |
5 |
- |
- |
3 |
|
|
Oxygen |
O |
8 |
8 |
8 |
8 |
2 |
6 |
- |
- |
2 |
|
|
Fluorine |
F |
9 |
9 |
10 |
9 |
2 |
7 |
- |
- |
1 |
|
|
Neon |
Ne |
10 |
10 |
10 |
10 |
2 |
8 |
- |
- |
0 |
|
|
Sodium |
Na |
11 |
11 |
12 |
10 |
2 |
8 |
1 |
- |
1 |
|
|
Magnesium |
Mg |
12 |
12 |
12 |
12 |
2 |
8 |
2 |
- |
2 |
|
|
Aluminium |
Al |
13 |
13 |
14 |
13 |
2 |
8 |
3 |
- |
3 |
|
|
Silicon |
Si |
14 |
14 |
14 |
14 |
2 |
8 |
4 |
- |
4 |
|
|
Phosphorus |
P |
15 |
15 |
16 |
15 |
2 |
8 |
5 |
- |
3 |
|
|
Sulphur |
S |
16 |
16 |
16 |
16 |
2 |
8 |
6 |
- |
2 |
|
|
Chlorine |
Cl |
17 |
17 |
18 |
17 |
2 |
8 |
7 |
- |
1 |
|
|
Argon |
Ar |
18 |
18 |
22 |
18 |
2 |
8 |
8 |
- |
0 |
|
Is atom an electrically neutral particle? If yes, why?
Atom is electrically neutral particle because in the nucleus of each atom the no. of protons present in the nucleus is equal to the no. of electrons revolving in its extra nuclear part. Each proton carries unit positive charge and each electron carries unit negative charge. Therefore, the no. of positive and negative charges in the atom are equal. This is why atom as a whole is electrically neutral particle.
How will you find the atomic mass of an element sodium and carbon? Illustrate your answer with examples.
Mass of an element can be obtained by adding the number of protons and neutrons. For examples:
|
Element |
Number of Protons |
Number of Neutrons |
Mass (u) |
|
Sodium Carbon |
11 6 |
12 6 |
23 12 |
An element has atomic number 15. Write the electronic configuration of the atom and indicate the number of shells it will occupy.
The atomic no. of the element is 15. So it contains 15 protons. Hence the atom contains 15 electrons. It will have the following electronic configuration
K L M
2 8 5
∴ The atom occupies three shells.
An atom contains three shells. What does it mean? It is possible that the same atom may have more shells?
An atom contains 3 shells, it means that in that atom the electrons are distributed broadly in three different energy levels. In the excited state the same atom may have the same electrons distributed in more than three energy levels and thus may have more shells.
Complete the following table:
|
Element |
Atomic number |
Protons |
Electrons |
Neutrons |
Mass number |
|
A B C |
17 — 9 |
9 — — |
14 — — |
18 14 10 |
19 — |
|
Element |
Atomic number |
Protons |
Electrons |
Neutrons |
Mass number |
|
A B C |
17 14 9 |
17 14 9 |
17 14 9 |
18 14 10 |
35 28 19 |
Sponsor Area
Atomic numbers and atomic mass numbers of the elements are given below. Complete the following information in the blanks:
| Elements | Atomic mass number | No. of protons | No. of neutrons | Electronic configuration |
| A | 28 | - | - | - |
| B | 20 | - | - | - |
| C | 23 | - | - | - |
| D | 35 | - | - | - |
| E | 40 | - | - | - |
| Elements | Atomic mass number | No. of protons | No. of neutrons | Electronic configuration |
| A | 28 | 14 | 14 | 2 8 4 |
| B | 20 | 10 | 10 | 2 8 |
| C | 23 | 11 | 12 | 2 8 1 |
| D | 35 | 17 | 18 | 2 8 7 |
| E | 40 | 20 | 20 | 2 8 8 2 |
The mass number of chlorine atom is 35 and its atomic number is 17. How will this chlorine atom be represented?
The mass number of chlorine and atomic number can be reprented as , ![]()
How does an atom become a cation?
On losing one or more electrons from its energy shell, an atom acquires positive charge and is called a cation. For example electronic configuration of Magnesium is K=2, L=8,M=2. On loosing 2 electron it become cation Mg2+.
What is an anion?
When an atom gains one or more electrons, it becomes negatively charged and called by the name of anion.
Substances from A to E have in them the distribution of electrons, neutrons and protons as follows:
|
Substances |
Electrons |
Neutrons |
Protons |
|
A B C D E |
4 8 18 17 17 |
4 9 22 20 18 |
3 9 18 17 17 |
Making use of these data find—
(i) a cation,
(ii) an anion,
(iii) atom of noble gas.
Cation — B (no. of protons > no. of electrons)
Anion — A (no. of electrons > no. of protons)
Noble gas — C (2, 8, 8).
What is meant by atomic no. of an element ? Does the atomic number of an element change when its atoms form ions ? Give one example each of diatomic and triatomic molecules.
Atomic number can be defined as the number of protons present in the nucleus of an atom. Atomic number of an element does not change when it forms ions because the number of protons does not alter. For example diatomic molecule—oxygen (O2) triatomic molecule- water (H2O), O3 (ozone), the atomic number of oxygen is 8 in all case.
What is the atomic number of an element which has 7 electrons in the M shell?
This means that K-shell and L-shell of the atom are completely filled. Now K-shell would have 2 electrons and L-shell would have 8 electrons.
So, total no. of electrons = 2 + 8 + 7= 17
Thus no. of protons = 17
∴
Atomic no. of the atom = 17.
Is there any change in atomic number when oxygen atoms form oxide ions?
No, atomic number does not change by forming compounds or ion. only number of electrons change.
The atomic numbers of aluminium and chlorine are 13 and 17 respectively. How many electrons are present in Al+3 and Cl- respectively?
Atomic no. of aluminium = 13
∴No. of protons in aluminium = 13
∴No. of electrons in aluminium = 13
Aluminium loses 3 electrons to give Al+3.
No. of electrons in Al+3 = 13 - 3 = 10
Thus,
Aluminium has 10 electron.
Similarly,
Atomic number of chlorine i=17
Chlorine gains one electron to give Cl-
No. of electrons in Cl- = 17 + 1 = 18.
Why is that certain atoms are radioactive while many others are not?
If the number of neutrons exceeds the number of protons in the atom, then becomes unstable. For example, ![]() containing 6 protons and 6 neutrons is stable while
containing 6 protons and 6 neutrons is stable while ![]() which contains 6 protons and 8 neutrons is unstable and is radioactive.
which contains 6 protons and 8 neutrons is unstable and is radioactive.
What kind of electronic configuration is attained by chemically inert substances?
Chemically inert substances except helium contain 8 electrons in their outermost shells i.e., outermost shells are completely filled. Helium has only two electrons in its outermost shell, but that is the maximum capacity of its outermost shell. Argon with atomic number 18 has the stable configuration : 2, 8, 8. Thus, substance whose atom has 8 electron in their outermost shell is a chemically inert substance.
If number of electrons in an atom is 8 and number of protons is also 8, then
(i) What is the atomic number of the atom ?
(ii) What is the charge on the atom?
(i) Atomic number defined as the number of protons present in nucleus. Also, number of protons are always equal to number of electron. Thus atomic number of atom is 8.
(ii)Since, there is no gain or loss of electron in atom. Therefore charge on the atom = 0.
Define the term isotope with a suitable example.
Atoms of the same element having the same atomic number but different atomic masses, are called isotopes. This means isotopes of an element have the same number of protons but different number of neutrons in their nuclei. For example, carbon has two isotopes.
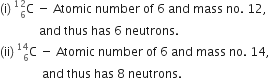
Two atoms of chlorine, A and B have the same nuclear charge. Their mass numbers are 35 and 37 respectively. What are the atoms collectively known as? What is the difference between the two ? Do they differ in their chemical properties?
(i) Two atoms having same nuclear charge have same no. of protons. But as atoms A and B have different mass numbers, these have different no. of neutrons. Such atoms are called isotopes.
(ii) They differ in their mass numbers and so have different physical properties.
(iii) They do not differ in their chemical properties—as they have same number of protons and electrons. The chemical properties of any element depend on the electrons.
State the similar properties of isotopes.
Properties of isotopes are:
(i) Isotopes of an element have same atomic number.
(ii) Isotopes of an element have similar chemical properties.
(iii) Isotopes of an element have similar no. of electrons.
(iv) Isotopes of an element have similar electronic configurations.
Examples:
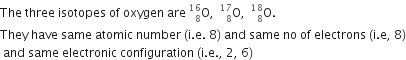
What are the uses of isotopes?
Isotopes have the following uses:
(i) Treatment of diseases—Radioactive isotopes are used for the treatment of dreadful diseases like cancer. Cobalt-60 is used to kill malignant cells in patients suffering from cancer.
(ii) Radioactive isotopes are used in chemical analysis.
(iii) Diseases in plants are investigated by using radioactive isotopes.
(iv) Production of energy : Uranium-235 can be subjected to fission process and thus production of electricity.
(v) Dating of plants, animals/human beings obtained from ancient times after excavation by using carbon-14.
Atomic mass of an element is the sum of no. of protons and neutrons which are whole numbers. How do you account for the fractional atomic masses of elements?
Ordinarly elements are composed of mixture of isotopes. For example, chlorine a mixture of
![]() and
and ![]()
Atomic mass of chlorine is the average of these two isotopes and is, therefore equal to
![]()
Composition of the nuclei of two atomic species A and B is given as under. Give the mass numbers of A and B. What is the relation between the two species and which element or elements do they represent
| A | B |
| Protons =6 | Protons=6 |
| Neutrons=6 | Neutrons =8 |
Mass number of A = 6 + 6 = 12
Atomic number of A = 6Composition of the nuclei of two atomic species A and B is given as under. Give the mass numbers of A and B. The relation between the two species and element represented as:
Mass number of B = 6 + 8 = 14
Atomic number of B = 6
Therefore, A and B have the same atomic number but different mass number.
So, Atoms of the same element i.e. carbon and are isotopes to each other.
The nuclear composition of two atomic species X and Y is![]()
What is the relation between these two atomic species ? Name the element or elements they represent.
Atomic mass of X = 8 + 8 = 16
Atomic number of X =8
Atomic mass of Y = 8 + 10 = 18
Atomic number of Y = 8
Since X and Y have the same atomic no. 8, but different mass nos. 16 and 18, they are atoms of the element, oxygen and are the isotopes to each other.
A and B are two atoms in whose nuclei, the number of protons and neutrons are:
|
Atoms |
Protons |
Neutrons |
|
A |
6 |
6 |
|
B |
6 |
7 |
Which element/elements do they represent and what is the relationship between them?
Mass number of A = 6 + 6 = 12
Mass number of B = 6 + 7 = 13
Atomic number is given by the number of protons present in atom. Since A and B have same number of proton number thus they having same atomic number but different mass number 12 and 13. They are atoms of carbon and are isotopes to each other.
Why do isotopes of an element differ in atomic masses and not in atomic number?
Isotopes of an element have the same number of protons and so are of the same atomic number. They differ in atomic masses due to different number of neutrons present in them.
The number of protons, neutrons and electrons in species from A to E are given in the following table.
|
Species |
Protons |
Neutrons |
Electrons |
|
A B C D E |
6 18 17 9 17 |
6 22 20 10 18 |
4 18 17 11 17 |
Indicate from the above table the species that represent:
(i) A cation
(ii) An anion
(iii) An atom of inert gas
(iv) A pair of isotopes.
(i) A is cation, since the number of electron is less than protons.
(ii) D is anion, since the number of electron is more than protons.
(iii) B is inert gas, since the outer electron is fully.The electronic configuration is 2,8,8 filled.
(iv) C and E are isotopes to each other, since the number of protons and electrons are same but differ in neutrons.
A, B and C are three elements. The nuclei of the atoms of these elements have protons and neutrons as under:
|
Atoms |
Protons |
Neutrons |
|
A |
1 |
0 |
|
B |
1 |
1 |
|
C |
1 |
2 |
Name the three elements and specify the relationship between them.
Mass number of A = 1 + 0 = 1
Mass number of B = 1 + 1 = 2
Mass number of C = 1 + 2 = 3
A, B and C are called protium
![]() Since the atomic number of A, B, C is the same, i.e., 1, these are isotopes to each other.
Since the atomic number of A, B, C is the same, i.e., 1, these are isotopes to each other.
Isotopes of elements have the same chemical properties but different physical properties. Give reasons.
Chemical properties of elements largely depend on their electronic configuration and as the isotopes of elements have similar electronic configurations, isotopes of elements have the same chemical properties. However, masses of isotopes of elements are different and therefore physical properties depending on mass such as density, light scattering etc. are different.
Sulphur exists as four naturally occurring isotopes with mass numbers 32, 33, 34, 35 and atomic number 16.
(i) Give atomic symbol for each indicating mass number and atomic number.
(ii) Would these have similar physical or chemical properties?
![]()
(ii) All the above substances are isotopes to each other and hence have similar chemical properties but would have different physical properties.
What are nucleons?
Protons and neutrons reside in the nucleus of an atom and are thus called nucleons. Since mass of an atom is due to protons and neutrons only, nucleons give the mass of atom. This is also called mass number.
What are isobars. Give examples.
Atoms of elements which have the same mass numbers but different atomic numbers are known as isobars. Atomic number of sodium is 11 and that of neon is 10. Mass number of both of them is 22.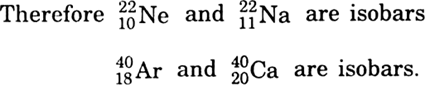
What do you say about physical and chemical properties of isobars?
Chemical properties depend on the electronic configuration. As atomic numbers of isobars are different, they have different electronic configurations and so different chemical properties. Further isobars have same mass. Hence they have same physical porperties.
Describe the essential features of the experiment that led to the discovery of isotopes.
The basic principles of mass spectrometer led to the discovery of isotopes. Ionized atoms of different masses are bent to different extent by the application of electric and magnetic field. From the extent of deflection and shapes of arcs photographed it is possible to determine the value of charge/mass ratio. Since the charge of the ionized atoms can be determined by techniques of electrolysis, the masses of the atoms can be determined. Thus elements with different masses can be detected and identified. It was realized that ionized atoms of many elements did not give the same mass. But the chemical properties of these atoms were identical. Such atoms were called isotopes.
For the symbol H, D and T tabulate three fundamental particles found in each of them.
|
Hydrogen (H) |
Deuterium (D) |
Tritium (T) |
|
|
Electron |
1 |
1 |
1 |
|
Proton |
1 |
1 |
1 |
|
Neutron |
0 |
1 |
2 |
Isotopes contain the same number of ___________ but different number of _________.
protons, neutrons
E. Rutherford used ____________ particles in his scattering experiments.
alpha
,Fill in the blanks:
E. Rutherford used ................ particles in his scattering experiments.
When α-particles are sent through a thin metal foil, most of them go straight through the foil because α-particles are _________.
most of the space is empty in atom.
The extranuclear part of the atom in which electrons are revolving are called _________.
orbits or energy levels
What happens when cathode rays strike a solid under suitable conditions?
When a cathode rays strikes a solid under a suitable condition, X-rays are produced.
Cathode rays cast shadow of the object placed in their path. What conclusion can be drawn from this observation regarding nature of cathode rays?
Cathode rays travel in straight lines.
Is the nature of anode rays independent of the gas used in the cathode rays tube like cathode rays?
No. It depends on the nature of gas.
Oxygen has three isotopes of atomic masses 16, 17 and 18 respectively. Comment on their electrical nature.
All the three are electrically neutral.
What part of the atom gives out alpha and beta particles during radioactivity?
Nucleus of the atom.
What must be added up to find the mass of an element:No. of protons and no. of neutrons or no. of electrons and no. of neutrons?
No. of neutrons and no. of protons should be added, in order to find mass of an atoml.
What is the maximum no. of electrons, an M-shell of the atom can accommodate?
18 electrons.
State the number of energy shells in an atom with atomic number 20.
There is Four energy shell in an atom having atomic number 20 as,
K=2
L=8
M=8
N=2.
Give the atomic number of the atom in which M-shell contains 4 electrons.
Since K and L is fully filled and M shell contain four electrons therefore total numbe of electron is 2+8+4= 14. Hence atomic number is 14.
When sodium atom (Na) changes to sodium ion (Na+), what is the change in atomic number?
There is no change in atomic number because number of protons remains constant.
The mass number of element A is 35. If its atomic number is 16. Give the number of neutrons.
Mass number of atom is given by total number of protons and neutrons. Since number of protons and mass number is given to us, thus
number of neutrons = mass number - protons
number of neutrons= 35-16 =19
An element has an atomic number of 21. How many electrons are there in each atom of the element?
Atom is smallest unit of element and the properties of every atom of element same.Since atomic number of element is 21 and number of electron is equal to number of protons thus there is 21 electrons in each atom.
Which of the following particles are equal in isotopes of an element : electrons, protons, neutrons?
Electrons and protons are equal in isotopes.
Who gave mass to charge ratio (e/ m) of an electron?
Thomson and Mullikan gave mass to charge ratio (e/m) of an electron.
Do isobars have similar chemical or physical properties?
Isobars of element have different chemical and physical properties.
Name the rays which are used to locate cracks in the fractured bones.
X-rays are used to locate cracks in the fractured bones.
Where are the valence electrons located in an atom?
Valence electrons in an atom are located in outermost energy shell or orbit of an atom.
α-particle is made up of
2 electrons and 2 protons
2 protons and 2 neutrons
2 electrons and 2 neutrons
helium atoms
B.
2 protons and 2 neutrons
An atom containing 16 electrons and 16 protons is represented as
16
18A35
16A0
18ACan't say
D.
Can't say
What is responsible for emission of different colours by different atoms?
Electronic configuration of atoms
Colour of atoms
Mass of atoms
Ratio of protons to neutrons in the atom
A.
Electronic configuration of atoms
When one neutron disintegrates, it produces
one proton and one electron
one proton only
one proton and one neutron
one electron and one neutron
A.
one proton and one electron
Electron was discovered by
J.J. Thomson
R.A. Mullikan
E. Rutherford
E. Goldstein
A.
J.J. Thomson
B.
R.A. Mullikan
C.
E. Rutherford
D.
E. Goldstein
Isotopes are
atoms of same element
atoms of different elements
molecules of same element
none of above
A.
atoms of same element
Isobars have
same number of protons and electrons
same number of protons and neutrons
same number of electrons and neutrons
same number of neutrons
D.
same number of neutrons
If an atom contains one electron and one proton, will it carry any charge or not?
An electron is a negatively charged particle, whereas a proton is a positively charged particle. The magnitude of their charges is equal. Therefore, an atom containing one electron and one proton will not carry any charge. Thus, it will be a neutral atom.
On the basis of Thomson's model of an atom, explain how the atom is neutral as a whole.
According to Thomson’s model of the atom, an atom consists of both negatively and positively charged particles. The negatively charged particles are embedded in the positively charged sphere. These negative and positive charges are equal in magnitude. Thus, by counterbalancing each other’s effect, they make an atom neutral.
On the basis of Rutherford's model of an atom, which subatomic particle is present in the nucleus of an atom?
On the basis of Rutherford's model of an atom, protons (positively-charged particles) are present in the nucleus of an atom.
What do you think would be at the observation if the ![]() -particle scattering experiment is carried out using a foil of a metal other than gold?
-particle scattering experiment is carried out using a foil of a metal other than gold?
If the ![]() scattering is carried out out using a foil of a metal rather than gold, there would be no change in the observation. In the α-scattering experiment, a gold foil was taken because gold is malleable and a thin foil of gold can be easily made. It is difficult to make such foils from other metals.
scattering is carried out out using a foil of a metal rather than gold, there would be no change in the observation. In the α-scattering experiment, a gold foil was taken because gold is malleable and a thin foil of gold can be easily made. It is difficult to make such foils from other metals.
Name the three sub-atomic particles of an atom.
The three sub-atomic particles of an atom are:
(i) Protons
(ii) Electrons, and
(iii) Neutrons
Helium atom has an atomic mass of 4 u and two protons in its nucleus. How many neutrons does it have?
Helium atom has two neutrons. The mass of an atom is the sum of the masses of protons and neutrons present in its nucleus. Since helium atom has two protons, mass contributed by the two protons is (2 × 1) u = 2 u. Then, the remaining mass (4 − 2) u = 2 u is contributed by 2u /1u = 2 neutrons.
Write the distribution of electrons in carbon and sodium atoms.
The total number of electrons in a carbon atom is 6. The distribution of electrons in carbon atom is given by:
First orbit or K-shell = 2 electrons
Second orbit or L-shell = 4 electrons
or, we can write the distribution of electrons in a carbon atom as 2, 4.
The total number of electrons in a sodium atom is 11. The distribution of electrons in sodium atom is given by:
First orbit or K-shell = 2 electrons
Second orbit or L-shell = 8 electrons
Third orbit or M-shell = 1 electron
or, we can write distribution of electrons in a sodium atom as 2, 8, and 1.
If K and L shells of an atom are full, then what would be the total number of electrons in the atom?
The maximum number of electrons that can occupy K and L-shells of an atom are 2 and 8 respectively. Therefore, if K and L-shells of an atom are full, then the total number of electrons in the atom would be (2 + 8) = 10 electrons.
How will you find the valency of chlorine, sulphur and magnesium?
If the number of electrons in the outermost shell of the atom of an element is less than or equal to 4, then the valency of the element is equal to the number of electrons in the outermost shell. On the other hand, if the number of electrons in the outermost shell of the atom of an element is greater than 4, then the valency of that element is determined by subtracting the number of electrons in the outermost shell from 8.
The distribution of electrons in chlorine, sulphur, and magnesium atoms are 2, 8, 7; 2, 8, 6 and 2, 8, 2 respectively.
Therefore, the number of electrons in the outer most shell of chlorine, sulphur, and magnesium atoms are 7, 6, and 2 respectively.
Thus, the valency of chlorine = 8 −7 = 1
The valency of sulphur = 8 − 6 = 2
The valency of magnesium = 2
If number of electrons in an atom is 8 and number of protons is also 8, then (i) what is the atomic number of the atom? and (ii) what is the charge on the atom?
(i) The atomic number is equal to the number of protons. Therefore, the atomic number of the atom is 8.
(ii) Since the number of both electrons and protons is equal, therefore, the charge on the atom is 0.
With the help of Table 4.1, find out the mass number of oxygen and sulphur atom.
Mass number of oxygen = Number of protons + Number of neutrons = 8 + 8 = 16
Mass number of sulphur = Number of protons + Number of neutrons = 16 +16 = 32
Mock Test Series
Sponsor Area
Sponsor Area