Sponsor Area
The Fundamental Unit Of Life
Describe Bohr's model of the atom ?
According to Bohr's theory:
(i) The atom consists of a small (positively charged) nucleus at its centre.
(ii) The whole mass of the atom is concentrated in the nucleus and the volume of the nucleus is smaller than the volume of the atom by a ratio of about 1 : 105.
(iii) All the protons and neutrons of the atom are contained in the nucleus.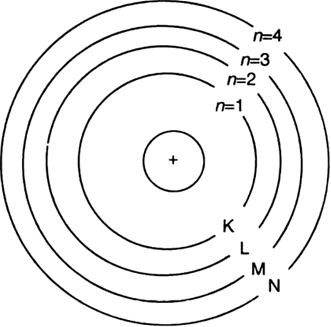
(iv) The electrons of the atom revolve around the nucleus in definite circular paths known as orbits or which are designated as K, L, M, N etc. or numbered as (n) = 1, 2, 3, 4 etc. outward from the nucleus.
(v) Each orbit is associated with a fixed amount of energy. Therefore, these orbits are also known as energy levels or energy shells.
(vi) The energy of the atom changes when an electron jumps from one state (energy level) to another state (energy level). As long as an electron remains in a particular orbit, it does not lose or gain energy.
Some More Questions From The Fundamental Unit of Life Chapter
Compare all the proposed models of an atom given in this chapter.
Summarize the rules for writing of distribution of electrons in various shells for the first eighteen elements.
Define valency by taking examples of silicon and oxygen.
Explain with examples, (i) Atomic number, (ii) Mass number, (iii) Isotopes, and (iv) Isobars. Give any two uses of isotopes also.
Na+ has completely filled K and L-shells. Explain.
If Z = 3, what would be the valency of the element? Also, name the element.
Composition of the nuclei of two atomic species X and Y are given as under
X Y
Protons = 6 6
Neutrons= 6 6
Give the mass numbers of X and Y. What is the relation between the two species?
X Y
Protons = 6 6
Neutrons= 6 6
Give the mass numbers of X and Y. What is the relation between the two species?
For the following statements, write T for True and F for False.
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
(b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
(c) The mass of an electron is about 1/2000 times that of proton.
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine.
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
(b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
(c) The mass of an electron is about 1/2000 times that of proton.
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine.
Rutherford’s α-particle scattering experiment was responsible for the discovery of
Sponsor Area
Mock Test Series
Mock Test Series





