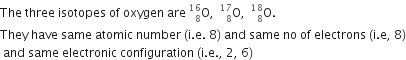State the similar properties of isotopes.
Properties of isotopes are:
(i) Isotopes of an element have same atomic number.
(ii) Isotopes of an element have similar chemical properties.
(iii) Isotopes of an element have similar no. of electrons.
(iv) Isotopes of an element have similar electronic configurations.
Examples: