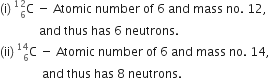Define the term isotope with a suitable example.
Atoms of the same element having the same atomic number but different atomic masses, are called isotopes. This means isotopes of an element have the same number of protons but different number of neutrons in their nuclei. For example, carbon has two isotopes.