Give one example for each of the following name reactions:
(i) Hell Volhard Zelinsky (HVZ) reaction,
(ii) Clemmensen’s reduction.
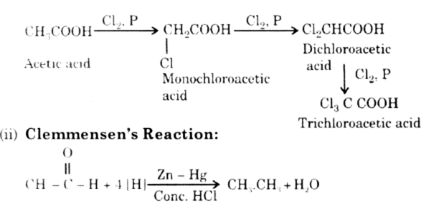
Give one example for each of the following name reactions:
(i) Hell Volhard Zelinsky (HVZ) reaction,
(ii) Clemmensen’s reduction.

Write balanced chemical equations for the following reactions:
(i) Oxalic acid is treated with acidified potassium permanganate solution.
(ii) Benzoic acid is treated with a mixture of concentrated nitric acid and concentrated sulphuric acid.
(iii) Methyl magnesium iodide is treated with carbon dioxide and the product hydrolysed in acidic medium.
(iii) Ethylacetate is treated with ammonia.
(i) Identify [A], [B], [C], [D] and [E],
(ii) Write balanced chemical equation of [D] with chlorine is the presence of red phosphorus and name the reaction. compounds relevant to these isomers.
When acetic acid is reacted with calcium hydroxide and the product is distilled dry, the compound formed is:
Write the structures of all enantiomers possible for lactic acid.
Carry out the following conversions:
(i) Methyl chloride to acetic acid.
(ii) Benzene to benzoic acid
(iii) Ethanol to acetone.
Give balanced equations for the following:
(i) Glycerol is heated with oxalic acid at 110° C (383K).
(ii) Acetamide is heated with sodium hydroxide.
(iii) Acetone reacts with hydrogen in the presence of heated copper.
Give an example (equation) for each of the following name reactions:
(i) Aldol condensation.
(ii) Reimer-Tiemann reaction.
(iii) Rosenmund’s reductions.
Give one example for each of the following name reactions:
(i) Hell Volhard Zelinsky (HVZ) reaction,
(ii) Clemmensen’s reduction.
Mock Test Series