Give balanced equations for the following:
(i) Glycerol is heated with oxalic acid at 110° C (383K).
(ii) Acetamide is heated with sodium hydroxide.
(iii) Acetone reacts with hydrogen in the presence of heated copper.
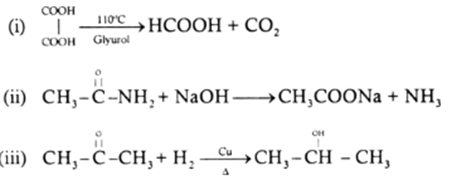
Give balanced equations for the following:
(i) Glycerol is heated with oxalic acid at 110° C (383K).
(ii) Acetamide is heated with sodium hydroxide.
(iii) Acetone reacts with hydrogen in the presence of heated copper.

Write the structures of all enantiomers possible for lactic acid.
Carry out the following conversions:
(i) Methyl chloride to acetic acid.
(ii) Benzene to benzoic acid
(iii) Ethanol to acetone.
Give balanced equations for the following:
(i) Glycerol is heated with oxalic acid at 110° C (383K).
(ii) Acetamide is heated with sodium hydroxide.
(iii) Acetone reacts with hydrogen in the presence of heated copper.
Give an example (equation) for each of the following name reactions:
(i) Aldol condensation.
(ii) Reimer-Tiemann reaction.
(iii) Rosenmund’s reductions.
Give one example for each of the following name reactions:
(i) Hell Volhard Zelinsky (HVZ) reaction,
(ii) Clemmensen’s reduction.
Give one good chemical test to distinguish between the following pairs of compounds:
(i) 1 Propanol and 2 Propanol.
(ii) Oxalic acid and benzoic acid.
Mock Test Series