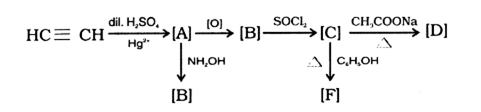An organic compound [A] having molecular formula C2H7N on treatment with nitrous acid gives a compound [B] having molecular formula C2H6O. [B] on treatment with an organic compound [C] gives a carboxylic acid [D] and a sweet smelling compound [E]. Oxidation of [B] with acidified potassium dichromate also gives [D].
(i) Identify [A], [B], [C], [D] and [E],
(ii) Write balanced chemical equation of [D] with chlorine is the presence of red phosphorus and name the reaction. compounds relevant to these isomers.
[A] → CH3CH2NH2 or Ethylamine
[B] → CH3CH2OH or Ethanol
[C] → (CH3CO2)O or Ethanoic anhydride
[D] → CH3COOH or Ethanoic acid
[E] → CH3COOC2H5 or Ethy ethanoate
ii) 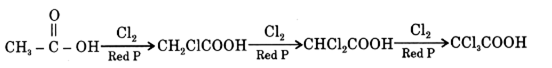
This reaction is called Hell-volhard -Zelinsky (HUZ) reaction.