Thermodynamics
Assertion: In an adiabatic process, change in internal energy of a gas is equal to work done on or by the gas in the process.
Reason: Temperature of gas remains constant in an adiabatic process.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
C.
If assertion is true but reason is false.
In an adiabatic process, no exchange of heat is permissible
i.e dQ = 0.
As dQ = dU + dW
= 0
∴ dU = - dW.
Assertion is true but reason is false.
Sponsor Area
Some More Questions From Thermodynamics Chapter
The work of 146 kJ is performed in order to compress one kilo mole of gas adiabatically and in this process the temperature of the gas increases by 7° C. The gas is
(R = 8.3 J mol−1 K−1 )
Which of the following is incorrect regarding the first law of thermodynamics?
A system goes from A to B via two processes I and II as shown in the figure. If ∆U1 and ∆U2 are the changes in internal energies in the processes I and II respectively, the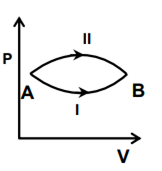
Which of the following statements is correct for any thermodynamic system?
Thermodynamic processes are indicated in the following diagram.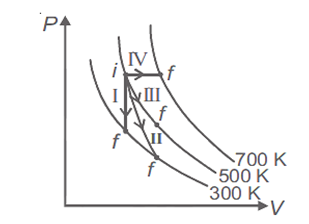
Match the following
| Column-1 | Column-2 |
| P. Process I | a. Adiabatic |
| Q. Process II | b. Isobaric |
| R. Process III | c. Isochoric |
| S. Process IV | d. Isothermal |
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area