Sponsor Area
Thermodynamics
Thermodynamic processes are indicated in the following diagram.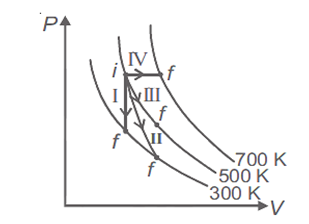
Match the following
| Column-1 | Column-2 |
| P. Process I | a. Adiabatic |
| Q. Process II | b. Isobaric |
| R. Process III | c. Isochoric |
| S. Process IV | d. Isothermal |
-
P → a, Q → c, R → d, S → b
-
P → c, Q → a, R → d, S → b
-
P → c, Q → d, R → b, S → a
-
P → d, Q → b, R → a, S
B.
P → c, Q → a, R → d, S → b
Process I = Isochoric
II = Adiabatic
III = Isothermal
IV = Isobaric
Some More Questions From Thermodynamics Chapter
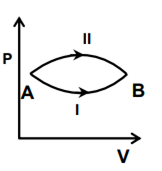
A system goes from A to B via two processes I and II as shown in the figure. If ∆U1 and ∆U2 are the changes in internal energies in the processes I and II respectively, the

Which of the following statements is correct for any thermodynamic system?
Thermodynamic processes are indicated in the following diagram.

Match the following
Column-1
Column-2
P. Process I
a. Adiabatic
Q. Process II
b. Isobaric
R. Process III
c. Isochoric
S. Process IV
d. Isothermal

Match the following
Sponsor Area
Mock Test Series
Mock Test Series





