Sponsor Area
Thermodynamics
A system goes from A to B via two processes I and II as shown in the figure. If ∆U1 and ∆U2 are the changes in internal energies in the processes I and II respectively, the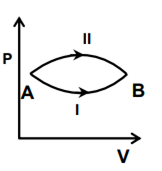
-
∆U1 = ∆U2
-
relation between ∆U1 and ∆U2 can not be determined
-
∆U2 > ∆U1
-
∆U2 < ∆U1
A.
∆U1 = ∆U2
Internal energy is state function
Some More Questions From Thermodynamics Chapter
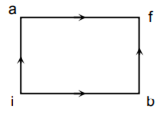
When a system is taken from state i to state f along the path iaf, it is found that Q = 50 cal and W = 20 cal. Along the path’ ibf Q = 36 cal. W along the path ibf is

The work of 146 kJ is performed in order to compress one kilo mole of gas adiabatically and in this process the temperature of the gas increases by 7° C. The gas is
(R = 8.3 J mol−1 K−1 )
(R = 8.3 J mol−1 K−1 )
Which of the following is incorrect regarding the first law of thermodynamics?
A system goes from A to B via two processes I and II as shown in the figure. If ∆U1 and ∆U2 are the changes in internal energies in the processes I and II respectively, the


Which of the following statements is correct for any thermodynamic system?
Sponsor Area
Mock Test Series
Mock Test Series





