Thermodynamics
When you make ice cubes, the entropy of water
does not change
increases
decreases
may either increase or decrease depeding on the process used
C.
decreases
The entropy function gives us a numerical measure of the irreversibility of a given process, ie, it is a measure of disorder of a system. During formation of ice cubes orderedness increases, ie, disorderness decreases, hence entropy decreases.
Sponsor Area
Some More Questions From Thermodynamics Chapter
Which of the following is incorrect regarding the first law of thermodynamics?
A system goes from A to B via two processes I and II as shown in the figure. If ∆U1 and ∆U2 are the changes in internal energies in the processes I and II respectively, the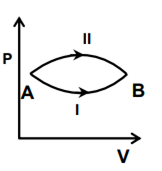
Which of the following statements is correct for any thermodynamic system?
Thermodynamic processes are indicated in the following diagram.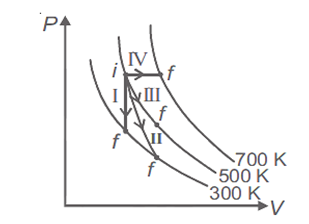
Match the following
| Column-1 | Column-2 |
| P. Process I | a. Adiabatic |
| Q. Process II | b. Isobaric |
| R. Process III | c. Isochoric |
| S. Process IV | d. Isothermal |
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area