Thermodynamics
In isothermal process, which of the following is not true?
Temperature remains constant
Internal energy does not change
No heat enters or leaves the system
none of the above
C.
No heat enters or leaves the system
In an isothermal process ( Temperature fixed), the ideal gas equation gives
PV = constant
i.e pressure of a given mass of gas varies inversely as its volume. In the isothermal process, heat enters or leaves the system, so keep the temperature constant, so statement (C) is wrong.
Sponsor Area
Some More Questions From Thermodynamics Chapter
A system goes from A to B via two processes I and II as shown in the figure. If ∆U1 and ∆U2 are the changes in internal energies in the processes I and II respectively, the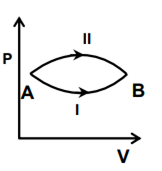
Which of the following statements is correct for any thermodynamic system?
Thermodynamic processes are indicated in the following diagram.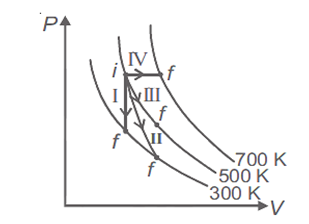
Match the following
| Column-1 | Column-2 |
| P. Process I | a. Adiabatic |
| Q. Process II | b. Isobaric |
| R. Process III | c. Isochoric |
| S. Process IV | d. Isothermal |
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area