The Fundamental Unit of Life
How were the results of discharge tube experiments interpreted by J. J. Thomson in terms of model of an atom?
The identification of cathode rays constituting charged electrons led to the conclusion that
(i) Electrons are present in an atom.
(ii) These electrons are embedded in a sphere of positive charge.
(iii) The comparison of e/m values for electron and H+, Na+ or other ions showed that the remaining part should be 2000 times heavier than the electron.
On this basis J.J. Thomson gave a model of the atom in which electrons and mass were distributed throughout the full size of atom. The size of the atom was estimated to be about 10-10 m. fig. gives model of three atoms containing one, two and three electrons. This model was considered to be very unstable.
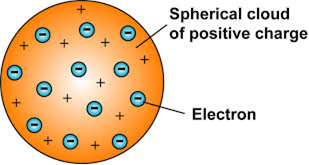
Thomson proposed the model of an atom to be similar to that of watermelon. the positive charge in the atom is spread all over like the red edible part of the watermelon, while the electrons are studded in the positively charged sphere, like the seeds in the watermelon.
Sponsor Area
Some More Questions From The Fundamental Unit of Life Chapter
Compare the properties of electron, proton and neutron.
What are the limitations of J.J. Thomson’s model of atom?
What are the limitations of Rutherford model of the atom?
Describe Bohr's model of the atom ?
Compare all the proposed models of an atom given in this chapter.
Summarize the rules for writing of distribution of electrons in various shells for the first eighteen elements.
Define valency by taking examples of silicon and oxygen.
Explain with examples, (i) Atomic number, (ii) Mass number, (iii) Isotopes, and (iv) Isobars. Give any two uses of isotopes also.
Na+ has completely filled K and L-shells. Explain.
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area