Coordination Compounds
Red precipitate is obtained when ethanol solution of dimethylglyoxime is added to ammoniacal Ni (II).Which of the following statements is not true?
-
Red Complex has a square planar geometry
-
Complex has symmetrical H- bonding
-
Red complex has a tetrahedral geometry
-
Dimethylglyoxime functions as bindentate ligand
C.
Red complex has a tetrahedral geometry
The reaction of Nickel with DMG Gives,
It shows that DMG acts as a bidentate ligand.
also, the geometry of DMG is square planar.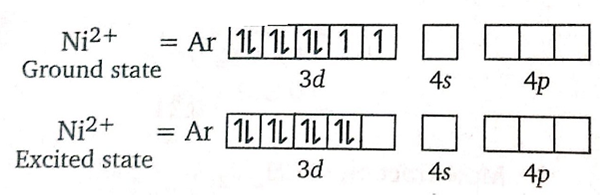
Hybridization of DMGi is dsp2 thus structure of is square planar.
Sponsor Area
Some More Questions From Coordination Compounds Chapter
Write the IUPAC names of the following coordination compounds:
(i) [Co(NH3)6]Cl3
(ii) [Co(NH3)5Cl]Cl2
(iii) K3[Fe(CN)6]
(iv) K3[Fe(C2O4)3]
(v) K2[PdCl4]
(vi) [Pt(NH3)2Cl(NH2CH3)]Cl.
Indicate the type of isomerism exhibited by the following complexes and draw structures for these isomers:
(i) K[Cr(H2O)2(C2O4)2], (ii) [Co(en)3Cl3,
(iii) [Co(NH3)5(NO2)]|NO3]2, (iv) [Pt(NH3)(H2O)Cl2]
Explain on the basis of valence bond theory that [Ni(CN)4]2– ion with square planar is diamagnetic and the [NiCl4]2– ion with tetrahedral geometry is paramagnetic.
Predict the number of unpaired electrons in the square planar [Pt(CN)4,]2– ion.
The hexaquo manganese(II) ion contains five unpaired electrons, while the hexacynoion contains only one unpaired electron. Explain using crystal field theory.
Calculate the overall complex dissociation equilibrium constant for the Cu(NH3)42+ion, given β4 for this complex is 2.1 x 1013.
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area