Coordination Compounds
Discuss the nature of bonding in the following coordination entities on the basis of valence bond theory.
[CoF6]3–
[CoF6]3– oxidation state of Co is +3
Fluorine ion is a weak ligand. It cannot cause the pairing of the 3d electrons. As a result, the Co3+ ion will undergo sp3d2 hybridzation.
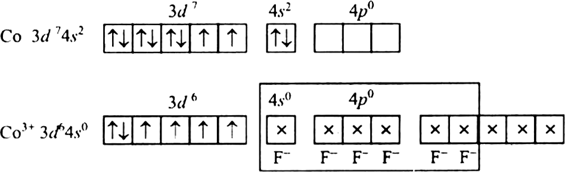
Hence, the geometry of the complex is octahedral and paramagnetic.
Sponsor Area
Some More Questions From Coordination Compounds Chapter
Write the IUPAC names of the following coordination compounds:
(i) [Co(NH3)6]Cl3
(ii) [Co(NH3)5Cl]Cl2
(iii) K3[Fe(CN)6]
(iv) K3[Fe(C2O4)3]
(v) K2[PdCl4]
(vi) [Pt(NH3)2Cl(NH2CH3)]Cl.
Indicate the type of isomerism exhibited by the following complexes and draw structures for these isomers:
(i) K[Cr(H2O)2(C2O4)2], (ii) [Co(en)3Cl3,
(iii) [Co(NH3)5(NO2)]|NO3]2, (iv) [Pt(NH3)(H2O)Cl2]
Explain on the basis of valence bond theory that [Ni(CN)4]2– ion with square planar is diamagnetic and the [NiCl4]2– ion with tetrahedral geometry is paramagnetic.
Predict the number of unpaired electrons in the square planar [Pt(CN)4,]2– ion.
The hexaquo manganese(II) ion contains five unpaired electrons, while the hexacynoion contains only one unpaired electron. Explain using crystal field theory.
Calculate the overall complex dissociation equilibrium constant for the Cu(NH3)42+ion, given β4 for this complex is 2.1 x 1013.
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area