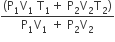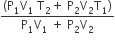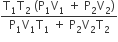Sponsor Area
Thermodynamics
An insulated container of gas has two chambers separated by an insulating partition. One of the chambers has volume V1 and contains ideal gas at pressure P1 and temperature T1. The other chamber has volume V2 and contains ideal gas at pressure P2 and temperature T2. If the partition is removed without doing any work on the gas, the final equilibrium temperature of the gas in the container will be
A.
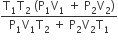
Some More Questions From Thermodynamics Chapter
A system goes from A to B via two processes I and II as shown in the figure. If ∆U1 and ∆U2 are the changes in internal energies in the processes I and II respectively, the
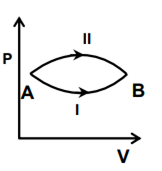

Which of the following statements is correct for any thermodynamic system?
Thermodynamic processes are indicated in the following diagram.
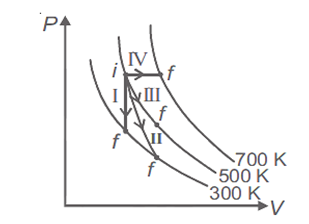
Match the following
Column-1
Column-2
P. Process I
a. Adiabatic
Q. Process II
b. Isobaric
R. Process III
c. Isochoric
S. Process IV
d. Isothermal

Match the following
Sponsor Area
Mock Test Series
Mock Test Series