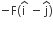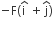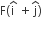Sponsor Area
NCERT Solutions for Class 11 Physics Physics Part I Chapter 7 System Of Particles And Rotational Motion
System Of Particles And Rotational Motion Here is the CBSE Physics Chapter 7 for Class 11 students. Summary and detailed explanation of the lesson, including the definitions of difficult words. All of the exercises and questions and answers from the lesson's back end have been completed. NCERT Solutions for Class 11 Physics System Of Particles And Rotational Motion Chapter 7 NCERT Solutions for Class 11 Physics System Of Particles And Rotational Motion Chapter 7 The following is a summary in Hindi and English for the academic year 2025-26. You can save these solutions to your computer or use the Class 11 Physics.
Determine the volume of 1 mole of any gas at S.T.P., assuming it behaves like an ideal Gas.
1 mole of any gas occupies 22.4 dm3 at stp (standard temperature and pressure, taken as 0°C and 1 atmosphere pressure). You may also have used a value of 24.0 dm3 at room temperature and pressure (taken as about 20°C and 1 atmosphere).
These figures are actually only true for an ideal gas, and we'll have a look at where they come from.
We can use the ideal gas equation to calculate the volume of 1 mole of an ideal gas at 0°C and 1 atmosphere pressure.
First, we have to get the units right.
0°C is 273 K. T = 273 K
1 atmosphere = 101325 Pa. p = 101325 Pa
We know that n = 1, because we are trying to calculate the volume of 1 mole of gas.
And, finally, R = 8.31441 J K-1 mol-1.
Slotting all of this into the ideal gas equation and then rearranging it gives:
And finally, because we are interested in the volume in cubic decimetres, you have to remember to multiply this by 1000 to convert from cubic metres into cubic decimetres.
The molar volume of an ideal gas is therefore 22.4 dm3 at stp.
And, of course, you could redo this calculation to find the volume of 1 mole of an ideal gas at room temperature and pressure - or any other temperature and pressure.
Sponsor Area
Mock Test Series
Mock Test Series






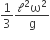
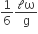
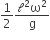
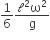

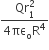
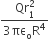
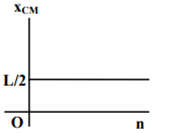
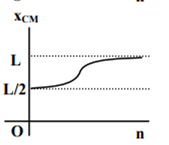
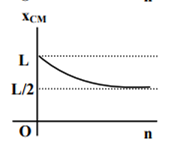
 where n can be zero or any positive number. If the position xCM of the centre of mass of the rod is plotted against ‘n’, which of the following graphs best approximates the dependence of xCM on n?
where n can be zero or any positive number. If the position xCM of the centre of mass of the rod is plotted against ‘n’, which of the following graphs best approximates the dependence of xCM on n? 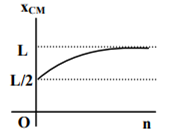
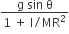
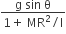
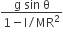
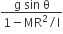
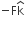 acts on O, the origin of the coordinate system. The torque about the point (1, −1) is
acts on O, the origin of the coordinate system. The torque about the point (1, −1) is