Sponsor Area
System Of Particles And Rotational Motion
- how does carnot cycle operates
- why does absolute zero not correspond to zero energy
- The Carnot cycle consists of the following four processes: A reversible isothermal gas expansion process. In this process, the ideal gas in the system absorbs qin amount heat from a heat source at a high temperature Th, expands and does work on surroundings.
-
As you know , absolute zero means the temperature in Kelvin scale is 0K or in Celcius scale is -273.15°C . actually, in this temperature, Gaseous molecule be rest , there is no motion of molecule in their position. due to this reason we say that energy of Gaseous molecule at this is zero. means absolute zero correspond to zero energy .
well, it's hard to achieve Absolute zero or 0K or -273.15°C temperature. it is just theoretical. molecular can't achieve Absolute zero.
Some More Questions From System of Particles and Rotational Motion Chapter
Angular momentum of the particle rotating with a central force is constant due to
Four point masses, each of value m, are placed at the corners of a square ABCD of side A. The moment of inertia through A and parallel to BD is
A wire elongates by
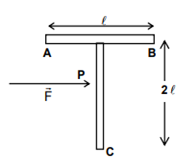
A ‘T’ shaped object with dimensions shown in the figure, is lying on a smooth floor. A force F is applied at the point P parallel to AB, such that the object has only the translational motion without rotation. Find the location of P with respect to C

Sponsor Area
Mock Test Series
Mock Test Series





