Thermodynamics
A lead ball at 30°C is dropped from a height of 6.2 km. The ball is heated due to the air resistance and it completely melts just before reaching the ground. The molten substance falls slowly on the ground. If the specific heat of lead = 126 Jkg-1 oC-1 and melting point of lead= 130°C and suppose that any mechanical energy lost is used to heat the ball, then the latent heat of fusion of lead is
2.4 × 104 J Kg-1
3.6 × 104 J Kg-1
7.6 × 102 J kg-1
4.94 × 103 J kg-1
D.
4.94 × 103 J kg-1
Given:-
Tm = 130o C
h = 6.2 km = 6200 m
S = 126 J kg-1 oC-1
Let the mass of the lead ball be m and its latent heat of fusion be L.
The initial temperature of the lead ball T1 = 30o C
The potential energy of the ball gets converted into heat melts the ball.
∴ mgh = mS ( Tm - T1 ) + mL
⇒ m (10) ( 6200 ) = m (126 ) (130 - 30 ) + mL
⇒ 6200 = 12600 + L
⇒ L = 4.94 × 104 J Kg-1
Sponsor Area
Some More Questions From Thermodynamics Chapter
Two moles of helium gas are taken over the cycle ABCDA, as shown in the P–T diagram. 
Assuming the gas to be ideal the work done on the gas in taking it from A to B is
When a system is taken from state i to state f along the path iaf, it is found that Q = 50 cal and W = 20 cal. Along the path’ ibf Q = 36 cal. W along the path ibf is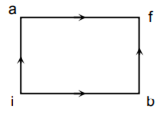
The work of 146 kJ is performed in order to compress one kilo mole of gas adiabatically and in this process the temperature of the gas increases by 7° C. The gas is
(R = 8.3 J mol−1 K−1 )
Which of the following is incorrect regarding the first law of thermodynamics?
A system goes from A to B via two processes I and II as shown in the figure. If ∆U1 and ∆U2 are the changes in internal energies in the processes I and II respectively, the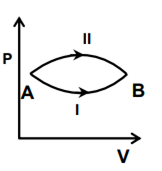
Which of the following statements is correct for any thermodynamic system?
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area