Thermodynamics
Question
Two points P and Q are maintained at the potential of 10V and -4V respectively. The work done in moving 100 electrons from P to Q is
-
9.60 × 10–17 J
-
9.60 × 10–17 J
-
– 2.24 × 10–16 J
-
2.24 × 10–16 J
Answer
D.
2.24 × 10–16 J
W = QdV = Q(Vq - VP)
= -100 × (1.6 × 10-19) × (– 4 – 10)
= + 100 × 1.6 × 10-19 × 14 = +2.24 10-16 J
Sponsor Area
Some More Questions From Thermodynamics Chapter
Which of the following statements is correct for any thermodynamic system?
Thermodynamic processes are indicated in the following diagram.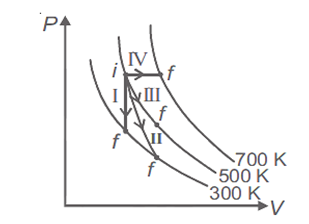
Match the following
| Column-1 | Column-2 |
| P. Process I | a. Adiabatic |
| Q. Process II | b. Isobaric |
| R. Process III | c. Isochoric |
| S. Process IV | d. Isothermal |
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area