Matter In Our Surroundings
Give reasons:
A gas exerts pressure on the walls of the container.
Particles of gas move randomly in all directions at high speed. As a result, the particles hit each other and also hit the walls of the container with a force. Therefore, gas exerts pressure on the walls of the container.
Sponsor Area
Some More Questions From Matter In Our Surroundings Chapter
What is the physical state of water at
a) 25°C
b)00C
c)1000C
What is the physical state of water at
0°C
What is the physical state of water at
100°C
Give two reasons to justify
Water at room temperature is a liquid.
Give two reasons to justify
An iron almirah is a solid at room temperature.
Why is ice at 273K more effective in cooling than water at the same temperature ?
What produces more severe burns, boiling water or steam ?
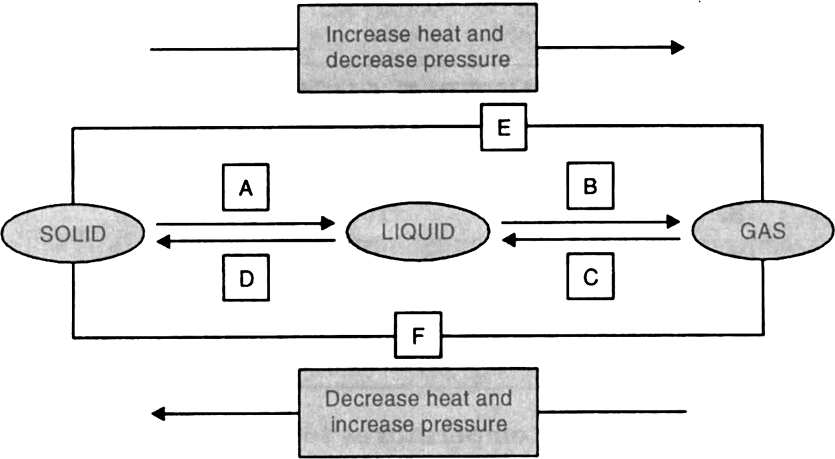
8. Name A, B, C, D, E and F in the following diagram showing state change :
Both boiling and evaporation convert a liquid into vapour. What is the difference between the two process
Define Evaporation
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area