Define Evaporation
The phenomenon of change of a liquid into vapours at any temperature below its boiling point is called evaporation. For example if Water left in open vessel at room temperature disappears after some time due to evaporation.
Sponsor Area
Define Evaporation
The phenomenon of change of a liquid into vapours at any temperature below its boiling point is called evaporation. For example if Water left in open vessel at room temperature disappears after some time due to evaporation.
Give reason for the following observations.
We can get the smell of perfume sitting several metres away.
What is the physical state of water at
a) 25°C
b)00C
c)1000C
What is the physical state of water at
0°C
What is the physical state of water at
100°C
Give two reasons to justify
Water at room temperature is a liquid.
Give two reasons to justify
An iron almirah is a solid at room temperature.
Why is ice at 273K more effective in cooling than water at the same temperature ?
What produces more severe burns, boiling water or steam ?
8. Name A, B, C, D, E and F in the following diagram showing state change :
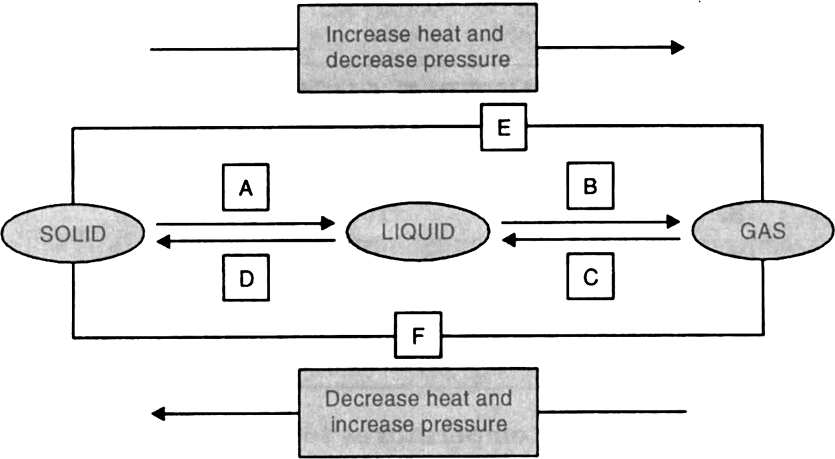
Both boiling and evaporation convert a liquid into vapour. What is the difference between the two process
Sponsor Area
Mock Test Series