Matter In Our Surroundings
Why is ice at 273K is less energetic than water at the same temperature
Cooling takes place when heat is removed from a system, in case of ice at 273 K, it will take latent heat from the medium to convert itself into water at 273K there will be a change in physical state, whereas in case of water at 273 K there will be no change in state. Hence lesser energy will be taken from the medium.
Sponsor Area
Some More Questions From Matter In Our Surroundings Chapter
What is the physical state of water at
a) 25°C
b)00C
c)1000C
What is the physical state of water at
0°C
What is the physical state of water at
100°C
Give two reasons to justify
Water at room temperature is a liquid.
Give two reasons to justify
An iron almirah is a solid at room temperature.
Why is ice at 273K more effective in cooling than water at the same temperature ?
What produces more severe burns, boiling water or steam ?
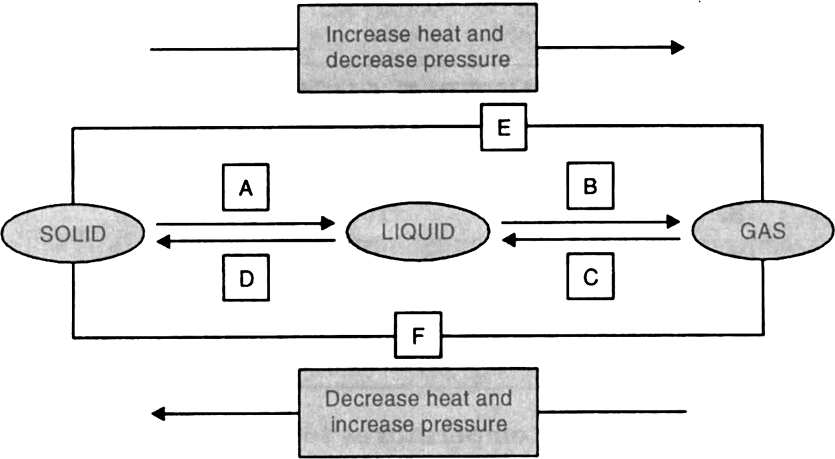
8. Name A, B, C, D, E and F in the following diagram showing state change :
Both boiling and evaporation convert a liquid into vapour. What is the difference between the two process
Define Evaporation
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area