Matter In Our Surroundings
Discuss the factors which affect evaporation
There are four factors which affect evaporation.
(a) Surface area. Evaporation is a surface phenomenon. Thus escaping of particles from liquid state to vapour state depends on surface area. Therefore, the rate of evaporation increases with surface area.
(b) Temperature of the system. With increase of surface temperature, the number of particles with larger kinetic energy increases and there is greater chance of escape of particles from liquid to vapour state. Therefore, rate of evaporation increases with temperature.
(c) Wind. More particles of liquid vapour would be carried away from the surface of the liquid with increasing speed of wind. Thus rate of evaporation increases with speed of wind.
(d) Humidity. Humidity is the amount of vapour present in the air. At a given temperature, air cannot hold more than a fixed amount of water vapour. Therefore, the rate of evaporation decreases with increase in the humidity of air.
Sponsor Area
Some More Questions From Matter In Our Surroundings Chapter
What is the physical state of water at
a) 25°C
b)00C
c)1000C
What is the physical state of water at
0°C
What is the physical state of water at
100°C
Give two reasons to justify
Water at room temperature is a liquid.
Give two reasons to justify
An iron almirah is a solid at room temperature.
Why is ice at 273K more effective in cooling than water at the same temperature ?
What produces more severe burns, boiling water or steam ?
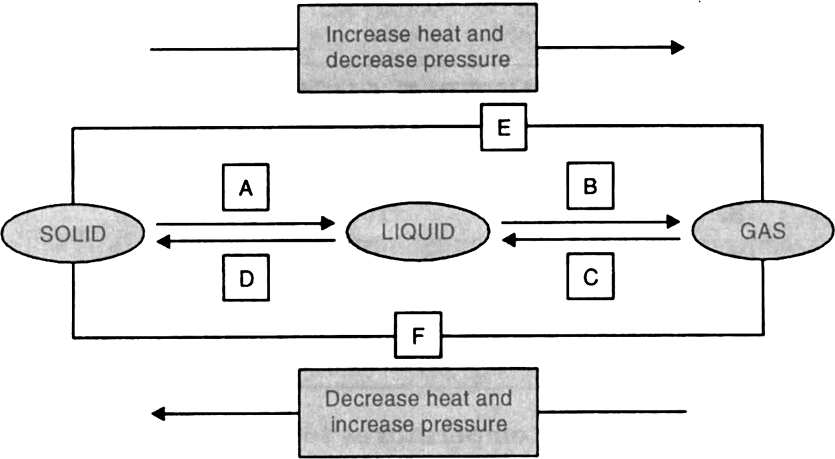
8. Name A, B, C, D, E and F in the following diagram showing state change :
Both boiling and evaporation convert a liquid into vapour. What is the difference between the two process
Define Evaporation
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area